A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - 
|
 |
 |
 |
 |
 |
 |
 |
 |
 3-4-diaminopyridine 3-4-diaminopyridine
|
 |
 |
mg |
23°C |
 |
| 180 |
 |
|
1675 |
 3-4-diaminopyridine 3-4-diaminopyridine
|
 |
 |
mg |
37°C |
 |
| 30 |
 |
|
1675 |
 3-4-diaminopyridine 3-4-diaminopyridine
|
 |
 |
mg |
4°C |
 |
| 180 |
 |
|
1675 |
 4-aminopyridine 4-aminopyridine
|
 |
 |
mg |
23°C |
 |
| 180 |
 |
|
1675 |
 4-aminopyridine 4-aminopyridine
|
 |
 |
mg |
37°C |
 |
| 30 |
 |
|
1675 |
 4-aminopyridine 4-aminopyridine
|
 |
 |
mg |
4°C |
 |
| 180 |
 |
|
1675 |
 5 aminosalicylic acid 5 aminosalicylic acid
|
 |
 |
mg |
22-24°C |
 |
| 24 |
 |
|
2524 |
 Acetazolamide Acetazolamide
|
 |
 |
0,375 mg/ml |
25°C |
 |
| 5 |
 |
|
460 |
 Aciclovir sodium Aciclovir sodium
|
 |
 |
5 mg/ml |
25°C |
 |
| 37 |
 |
|
68 |
 Aciclovir sodium Aciclovir sodium
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 63 |
 |
|
4573 |
 Aciclovir sodium Aciclovir sodium
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 21 |
 |
|
3001 |
 Aciclovir sodium Aciclovir sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 30 |
 |
|
1717 |
 Aciclovir sodium Aciclovir sodium
|
 |
 |
1 & 10 mg/ml |
25°C |
 |
| 4 |
 |
|
604 |
 Adalimumab Adalimumab
|
 |
 |
100 mg/ml |
25°C |
 |
| 28 |
 |
|
4644 |
 Adalimumab Adalimumab
|
 |
 |
50 mg/ml |
25°C |
 |
| 28 |
 |
|
4644 |
 Adenosin Adenosin
|
 |
 |
0,05 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3223 |
 Adenosin Adenosin
|
 |
 |
0,05 mg/ml |
23-25°C |
 |
| 14 |
 |
|
3223 |
 Adenosin Adenosin
|
 |
 |
0,1 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3223 |
 Adenosin Adenosin
|
 |
 |
0,1 mg/ml |
23-25°C |
 |
| 14 |
 |
|
3223 |
 Adenosin Adenosin
|
 |
 |
0,22 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3223 |
 Adenosin Adenosin
|
 |
 |
0,22 mg/ml |
23-25°C |
 |
| 14 |
 |
|
3223 |
 Adenosin Adenosin
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4070 |
 Adenosin Adenosin
|
 |
 |
2 mg/ml |
20-25°C |
 |
| 14 |
 |
|
4070 |
 Adenosin Adenosin
|
 |
 |
0,01 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3422 |
 Adenosin Adenosin
|
 |
 |
0,01 mg/ml |
20-25°C |
 |
| 14 |
 |
|
3422 |
 Adenosin Adenosin
|
 |
 |
0,05 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3422 |
 Adenosin Adenosin
|
 |
 |
0,05 mg/ml |
20-25°C |
 |
| 14 |
 |
|
3422 |
 Adenosin Adenosin
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4070 |
 Adenosin Adenosin
|
 |
 |
2 mg/ml |
20-25°C |
 |
| 14 |
 |
|
4070 |
 Aflibercept Aflibercept
|
 |
 |
0,6 >> 8 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3508 |
 Aflibercept Aflibercept
|
 |
 |
0,6 >> 8 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3508 |
 Aldesleukin Aldesleukin
|
 |
 |
18 MUI/ml |
30°C |
 |
| 48 |
 |
|
3635 |
 Alpha-tocopherol acetate Alpha-tocopherol acetate
|
 |
 |
mg |
25°C |
 |
| 60 |
 |
|
2988 |
 Alprostadil Alprostadil
|
 |
 |
25 µg/ml |
4°C |
 |
| 82 |
 |
|
2003 |
 Alteplase Alteplase
|
 |
 |
> 0,2 mg/ml |
25°C |
 |
| 8 |
 |
|
3616 |
 Amifostine Amifostine
|
 |
 |
50 mg/ml |
25°C |
 |
| 6 |
 |
|
3598 |
 Amikacin sulfate Amikacin sulfate
|
 |
 |
0,25 & 5 mg/ml |
25°C |
 |
| 24 |
 |
|
561 |
 Amikacin sulfate Amikacin sulfate
|
 |
 |
4 mg/ml |
25°C |
 |
| 48 |
 |
|
565 |
 Amikacin sulfate Amikacin sulfate
|
 |
 |
0,25 & 5 mg/ml |
25°C |
 |
| 24 |
 |
|
561 |
 Amikacin sulfate Amikacin sulfate
|
 |
 |
0,25 & 5 mg/ml |
25°C |
 |
| 24 |
 |
|
561 |
 Amikacin sulfate Amikacin sulfate
|
 |
 |
0,25 & 20 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Aminocaproic acid Aminocaproic acid
|
 |
 |
10 & 100 mg/ml |
23°C |
 |
| 7 |
 |
|
550 |
 Aminophylline Aminophylline
|
 |
 |
5 mg/ml |
22°C |
 |
| 91 |
 |
|
478 |
 Aminophylline Aminophylline
|
 |
 |
5 mg/ml |
4°C |
 |
| 91 |
 |
|
478 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
0,6 mg/ml |
22°C-24°C |
 |
| 5 |
 |
|
379 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 42 |
 |
|
2541 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 42 |
 |
|
3792 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
4°C |
 |
| 91 |
 |
|
2541 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
4°C |
 |
| 91 |
 |
|
2541 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
4°C |
 |
| 91 |
 |
|
3792 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
2-8°C |
 |
| 91 |
 |
|
3810 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 42 |
 |
|
2541 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 91 |
 |
|
3810 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 91 |
 |
|
3792 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
mg/ml |
4°C |
 |
| 91 |
 |
|
3792 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
0.125 mg/ml |
25°C |
 |
| 48 |
 |
|
3777 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
0.125 mg/ml |
37°C |
 |
| 24 |
 |
|
3777 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
0.125 mg/ml |
4°C |
 |
| 14 |
 |
|
3777 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
0.125 mg/ml |
25°C |
 |
| 72 |
 |
|
3777 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
0.125 mg/ml |
37°C |
 |
| 24 |
 |
|
3777 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
0.125 mg/ml |
4°C |
 |
| 14 |
 |
|
3777 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
25 mg/ml |
25°C |
 |
| 12 |
 |
|
4211 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
25 mg/ml |
4-8°C |
 |
| 24 |
 |
|
4211 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
10 & 20 mg/ml |
25°C |
 |
| 8 |
 |
|
1333 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 3 |
 |
|
1333 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
10 & 20 mg/ml |
25°C |
 |
| 8 |
 |
|
1333 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 3 |
 |
|
1333 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 2 |
 |
|
1333 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 1 |
 |
|
1333 |
 Amoxicillin sodium / clavulanic acid Amoxicillin sodium / clavulanic acid
|
 |
 |
mg/ml |
25°C |
 |
| 7 |
 |
|
2508 |
 Amoxicillin sodium / clavulanic acid Amoxicillin sodium / clavulanic acid
|
 |
 |
mg/ml |
25°C |
 |
| 7 |
 |
|
2508 |
 Amoxicillin sodium / clavulanic acid Amoxicillin sodium / clavulanic acid
|
 |
 |
mg/ml |
5°C |
 |
| 10 |
 |
|
2508 |
 Amoxicillin sodium / clavulanic acid Amoxicillin sodium / clavulanic acid
|
 |
 |
mg/ml |
5°C |
 |
| 10 |
 |
|
2508 |
 Amoxicillin sodium / clavulanic acid Amoxicillin sodium / clavulanic acid
|
 |
 |
mg/ml |
8°C |
 |
| 7 |
 |
|
2951 |
 Amphotericin B Amphotericin B
|
 |
 |
0,92 >> 1,4 mg/ml |
25°C |
 |
| 36 |
 |
|
439 |
 Amphotericin B Amphotericin B
|
 |
 |
mg/ml |
23-27°C |
 |
| 28 |
 |
|
4487 |
 Amphotericin B Amphotericin B
|
 |
 |
mg/ml |
3-7°C |
 |
| 168 |
 |
|
4487 |
 Amphotericin B liposomale Amphotericin B liposomale
|
 |
 |
4 mg/ml |
4°C |
 |
| 7 |
 |
|
3398 |
 Amphotericin B liposomale Amphotericin B liposomale
|
 |
 |
4 mg/ml |
4°C |
 |
| 7 |
 |
|
3398 |
 Amphotericin B liposomale Amphotericin B liposomale
|
 |
 |
0,2 >> 2 mg/ml |
2-8°C |
 |
| 7 |
 |
|
3398 |
 Amphotericin B liposomale Amphotericin B liposomale
|
 |
 |
0,2 >> 2 mg/ml |
23-27°C |
 |
| 72 |
 |
|
3398 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
0.125 mg/ml |
25°C |
 |
| 48 |
 |
|
3777 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
0.125 mg/ml |
37°C |
 |
| 24 |
 |
|
3777 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
0.125 mg/ml |
4°C |
 |
| 14 |
 |
|
3777 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
0.125 mg/ml |
25°C |
 |
| 72 |
 |
|
3777 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
0.125 mg/ml |
37°C |
 |
| 24 |
 |
|
3777 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
0.125 mg/ml |
4°C |
 |
| 14 |
 |
|
3777 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
20 & 30 mg/ml |
25°C |
 |
| 8 |
 |
|
604 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
12 mg/ml |
25°C |
 |
| 24 |
 |
|
3880 |
 Ampicillin sodium - sulbactam sodium Ampicillin sodium - sulbactam sodium
|
 |
 |
20 mg/ml |
24°C |
 |
| 24 |
 |
|
258 |
 Ampicillin sodium - sulbactam sodium Ampicillin sodium - sulbactam sodium
|
 |
 |
20 mg/ml |
5°C |
 |
| 60 |
 |
|
258 |
 Amsacrine Amsacrine
|
 |
 |
1,5 mg/ml |
25°C |
 |
| 5 |
 |
|
3576 |
 Anidulafungin Anidulafungin
|
 |
 |
3.33 mg/ml |
25°C |
 |
| 24 |
 |
|
3567 |
 Anidulafungin Anidulafungin
|
 |
 |
0.77 mg/ml |
25°C |
 |
| 48 |
 |
|
3567 |
 Apomorphine Apomorphine
|
 |
 |
mg/ml |
2-8°C |
 |
| 24 |
 |
|
3615 |
 Apomorphine Apomorphine
|
 |
 |
mg/ml |
25°C |
 |
| 24 |
 |
|
3615 |
 Apomorphine Apomorphine
|
 |
 |
mg/ml |
2-8°C |
 |
| 24 |
 |
|
3615 |
 Apomorphine Apomorphine
|
 |
 |
mg/ml |
25°C |
 |
| 24 |
 |
|
3615 |
 Arsenic trioxyde Arsenic trioxyde
|
 |
 |
? mg/ml |
15-25°C |
 |
| 30 |
 |
|
4726 |
 Arsenic trioxyde Arsenic trioxyde
|
 |
 |
? mg/ml |
2-8°C |
 |
| 30 |
 |
|
4726 |
 Artesunate Artesunate
|
 |
 |
mg |
22°C |
 |
| 3 |
 |
|
2655 |
 Asparaginase Asparaginase
|
 |
 |
UI/ml |
4°C |
 |
| 7 |
 |
|
2148 |
 Asparaginase Asparaginase
|
 |
 |
? UI/ml |
8°C |
 |
| 7 |
 |
|
1858 |
 Asparaginase Asparaginase
|
 |
 |
? UI/ml |
8°C |
 |
| 7 |
 |
|
1858 |
 Asparaginase Asparaginase
|
 |
 |
? UI/ml |
8°C |
 |
| 7 |
 |
|
1858 |
 Asparaginase Asparaginase
|
 |
 |
? UI/ml |
8°C |
 |
| 7 |
 |
|
1858 |
 Atezolizumab Atezolizumab
|
 |
 |
3.2 >> 16.8 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4532 |
 Atezolizumab Atezolizumab
|
 |
 |
3.2 >> 16.8 mg/ml |
<30°C |
 |
| 24 |
 |
|
4532 |
 Atezolizumab Atezolizumab
|
 |
 |
3.2 >> 16.8 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4532 |
 Atezolizumab Atezolizumab
|
 |
 |
3.2 >> 16.8 mg/ml |
<30°C |
 |
| 24 |
 |
|
4532 |
 Atezolizumab Atezolizumab
|
 |
 |
3.2 >> 16.8 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4532 |
 Atezolizumab Atezolizumab
|
 |
 |
3.2 >> 16.8 mg/ml |
<30°C |
 |
| 24 |
 |
|
4532 |
 Atezolizumab Atezolizumab
|
 |
 |
3.2 >> 16.8 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4532 |
 Atezolizumab Atezolizumab
|
 |
 |
3.2 >> 16.8 mg/ml |
<30°C |
 |
| 24 |
 |
|
4532 |
 Atracurium besylate Atracurium besylate
|
 |
 |
10 mg/ml |
25°C |
 |
| 42 |
 |
|
657 |
 Atracurium besylate Atracurium besylate
|
 |
 |
10 mg/ml |
40°C |
 |
| 7 |
 |
|
657 |
 Azacitidine Azacitidine
|
 |
 |
10 & 25 mg/ml |
25°C |
 |
| 2 |
 |
|
4227 |
 Azacitidine Azacitidine
|
 |
 |
25 mg/ml |
2-8°C |
 |
| 8 |
 |
|
3236 |
 Azacitidine Azacitidine
|
 |
 |
25 mg/ml |
25°C |
 |
| 0.75 |
 |
|
3236 |
 Azacitidine Azacitidine
|
 |
 |
0.2 mg/ml |
25°C |
 |
| 1.9 |
 |
|
1039 |
 Azacitidine Azacitidine
|
 |
 |
2 mg/ml |
25°C |
 |
| 2.4 |
 |
|
1039 |
 Azacitidine Azacitidine
|
 |
 |
0.2 mg/ml |
25°C |
 |
| 1.9 |
 |
|
1039 |
 Azacitidine Azacitidine
|
 |
 |
2 mg/ml |
25°C |
 |
| 2.8 |
 |
|
1039 |
 Azacitidine Azacitidine
|
 |
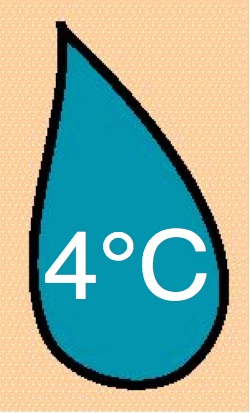 |
10 & 25 mg/ml |
-20°C |
 |
| 21 |
 |
|
4227 |
 Azacitidine Azacitidine
|
 |
 |
10 & 25 mg/ml |
25°C |
 |
| 4 |
 |
|
4227 |
 Azacitidine Azacitidine
|
 |
 |
10 & 25 mg/ml |
4°C |
 |
| 24 |
 |
|
4227 |
 Azacitidine Azacitidine
|
 |
 |
1.4 >> 2.4 mg/ml |
4°C |
 |
| 3 |
 |
|
3816 |
 Azacitidine Azacitidine
|
 |
 |
10 & 25 mg/ml |
-20°C |
 |
| 21 |
 |
|
4227 |
 Azacitidine Azacitidine
|
 |
 |
10 & 25 mg/ml |
4°C |
 |
| 24 |
 |
|
4227 |
 Azacitidine Azacitidine
|
 |
 |
0.5 & 2 mg/ml |
20°C |
 |
| 2 |
 |
|
414 |
 Azacitidine Azacitidine
|
 |
 |
25 mg/ml |
-20°C |
 |
| 23 |
 |
|
3351 |
 Aztreonam Aztreonam
|
 |
 |
60 mg/ml |
37°C |
 |
| 24 |
 |
|
1064 |
 Aztreonam Aztreonam
|
 |
 |
10 & 20 mg/ml |
25°C |
 |
| 48 |
 |
|
422 |
 Aztreonam Aztreonam
|
 |
 |
10 mg/ml |
24°C |
 |
| 4 |
 |
|
258 |
 Aztreonam Aztreonam
|
 |
 |
10 mg/ml |
5°C |
 |
| 4 |
 |
|
258 |
 Aztreonam Aztreonam
|
 |
 |
5 & 20 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Aztreonam Aztreonam
|
 |
 |
100 mg/ml |
37°C |
 |
| 24 |
 |
|
2042 |
 Baclofen Baclofen
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Baclofen Baclofen
|
 |
 |
3 mg/ml |
25°C |
 |
| 1095 |
 |
|
3969 |
 Baclofen Baclofen
|
 |
 |
3 mg/ml |
37°C |
 |
| 219 |
 |
|
3969 |
 Belatacept Belatacept
|
 |
 |
2 >> 10 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3261 |
 Belatacept Belatacept
|
 |
 |
2 >> 10 mg/ml |
25°C |
 |
| 4 |
 |
|
3261 |
 Belimumab Belimumab
|
 |
 |
? mg/ml |
15-25°C |
 |
| 8 |
 |
|
3936 |
 Belimumab Belimumab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 8 |
 |
|
3936 |
 Belimumab Belimumab
|
 |
 |
? mg/ml |
15-25°C |
 |
| 8 |
 |
|
3936 |
 Belimumab Belimumab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 8 |
 |
|
3936 |
 Belinostat Belinostat
|
 |
 |
50 mg/ml |
15-25°C |
 |
| 12 |
 |
|
3721 |
 Belinostat Belinostat
|
 |
 |
8 mg/ml |
15-25°C |
 |
| 36 |
 |
|
3721 |
 Bendamustine hydrochloride Bendamustine hydrochloride
|
 |
 |
≈ 0.3 >> 0.6 mg/ml |
2-8°C |
 |
| 48 |
 |
|
3158 |
 Bendamustine hydrochloride Bendamustine hydrochloride
|
 |
 |
≈ 0.3 >> 0.6 mg/ml |
25°C |
 |
| 3.5 |
 |
|
3158 |
 Bendamustine hydrochloride Bendamustine hydrochloride
|
 |
 |
0,25 mg/ml |
23°C |
 |
| 9 |
 |
|
2289 |
 Bevacizumab Bevacizumab
|
 |
 |
1,4 & 16,5 mg/ml |
2-8°C |
 |
| 42 |
 |
|
4756 |
 Bevacizumab Bevacizumab
|
 |
 |
1,4 >>16,5 mg/ml |
2-8°C |
 |
| 35 |
 |
|
4516 |
 Bevacizumab Bevacizumab
|
 |
 |
1.4 >> 16.5 mg/ml |
2-30°C |
 |
| 48 |
 |
|
4467 |
 Bevacizumab Bevacizumab
|
 |
 |
1.4 >> 16.5 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4467 |
 Bevacizumab Bevacizumab
|
 |
 |
1,4 & 16,5 mg/ml |
2-8°C |
 |
| 42 |
 |
|
4756 |
 Bevacizumab Bevacizumab
|
 |
 |
1,4 & 16,5 mg/ml |
25°C |
 |
| 3 |
 |
|
4714 |
 Bevacizumab Bevacizumab
|
 |
 |
1,4 >>16,5 mg/ml |
2-8°C |
 |
| 35 |
 |
|
4516 |
 Bevacizumab Bevacizumab
|
 |
 |
1.4 >> 16.5 mg/ml |
2-30°C |
 |
| 48 |
 |
|
4467 |
 Bevacizumab Bevacizumab
|
 |
 |
1.4 >> 16.5 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4467 |
 Bevacizumab Bevacizumab
|
 |
 |
16 mg/ml |
22°C |
 |
| 90 |
 |
|
2882 |
 Bevacizumab Bevacizumab
|
 |
 |
16 mg/ml |
4°C |
 |
| 90 |
 |
|
2882 |
 Bevacizumab Bevacizumab
|
 |
 |
2 mg/ml |
22°C |
 |
| 90 |
 |
|
2882 |
 Bevacizumab Bevacizumab
|
 |
 |
2 mg/ml |
4°C |
 |
| 90 |
 |
|
2882 |
 Bevacizumab Bevacizumab
|
 |
 |
1,4 & 16,5 mg/ml |
2-8°C |
 |
| 42 |
 |
|
4756 |
 Bevacizumab Bevacizumab
|
 |
 |
25 mg/ml |
2-8°C |
 |
| 180 |
 |
|
2661 |
 Bevacizumab Bevacizumab
|
 |
 |
25 mg/ml |
2-8°C |
 |
| 180 |
 |
|
2661 |
 Bivalirudin Bivalirudin
|
 |
 |
50 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3539 |
 Bivalirudin Bivalirudin
|
 |
 |
5 mg/ml |
25°C |
 |
| 24 |
 |
|
3539 |
 Bleomycin sulfate Bleomycin sulfate
|
 |
 |
0,3 & 3 mg/ml |
22°C-25°C |
 |
| 24 |
 |
|
252 |
 Bleomycin sulfate Bleomycin sulfate
|
 |
 |
3 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3646 |
 Bleomycin sulfate Bleomycin sulfate
|
 |
 |
0,3 & 3 mg/ml |
22°C-25°C |
 |
| 24 |
 |
|
252 |
 Blinatumomab Blinatumomab
|
 |
 |
0,26 µg/ml |
2-8°C |
 |
| 10 |
 |
|
3774 |
 Blinatumomab Blinatumomab
|
 |
 |
0,26 µg/ml |
23-27°C |
 |
| 96 |
 |
|
3774 |
 Blinatumomab Blinatumomab
|
 |
 |
0,26 µg/ml |
2-8°C |
 |
| 10 |
 |
|
3774 |
 Blinatumomab Blinatumomab
|
 |
 |
0,26 µg/ml |
23-27°C |
 |
| 96 |
 |
|
3774 |
 Bortezomib Bortezomib
|
 |
 |
2.5 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3537 |
 Bortezomib Bortezomib
|
 |
 |
2.5 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3537 |
 Busulfan Busulfan
|
 |
 |
0,1 mg/ml |
23°C |
 |
| 4 |
 |
|
658 |
 Busulfan Busulfan
|
 |
 |
0,5 mg/ml |
23°C |
 |
| 8 |
 |
|
658 |
 Busulfan Busulfan
|
 |
 |
0,5 mg/ml |
23°C |
 |
| 8 |
 |
|
658 |
 Busulfan Busulfan
|
 |
 |
0,12 mg/ml |
25°C |
 |
| 12 |
 |
|
3198 |
 Busulfan Busulfan
|
 |
 |
0,12 mg/ml |
4°C |
 |
| 12 |
 |
|
3198 |
 Busulfan Busulfan
|
 |
 |
0,24 mg/ml |
25°C |
 |
| 8 |
 |
|
3198 |
 Busulfan Busulfan
|
 |
 |
0,24 mg/ml |
4°C |
 |
| 16 |
 |
|
3198 |
 Busulfan Busulfan
|
 |
 |
0,1 mg/ml |
23°C |
 |
| 4 |
 |
|
658 |
 Busulfan Busulfan
|
 |
 |
6 mg/ml |
4°C |
 |
| 4 |
 |
|
3285 |
 Cabazitaxel Cabazitaxel
|
 |
 |
10 mg/ml |
-20°C |
 |
| 49 |
 |
|
4800 |
 Cabazitaxel Cabazitaxel
|
 |
 |
0,1 mg/ml |
-20°C |
 |
| 49 |
 |
|
4800 |
 Cabazitaxel Cabazitaxel
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 21 |
 |
|
4800 |
 Cabazitaxel Cabazitaxel
|
 |
 |
0,1 mg/ml |
4°C |
 |
| 21 |
 |
|
4800 |
 Cabazitaxel Cabazitaxel
|
 |
 |
0,10 >> 0,26 mg/ml |
2-8°C |
 |
| 48 |
 |
|
3237 |
 Cabazitaxel Cabazitaxel
|
 |
 |
0,10 >> 0,26 mg/ml |
25°C |
 |
| 8 |
 |
|
3237 |
 Cabazitaxel Cabazitaxel
|
 |
 |
0,26 mg/ml |
-20°C |
 |
| 49 |
 |
|
4800 |
 Cabazitaxel Cabazitaxel
|
 |
 |
0,26 mg/ml |
25°C |
 |
| 21 |
 |
|
4800 |
 Cabazitaxel Cabazitaxel
|
 |
 |
0,26 mg/ml |
4°C |
 |
| 21 |
 |
|
4800 |
 Caffeine Caffeine
|
 |
 |
10 mg/ml |
25°C |
 |
| 60 |
 |
|
990 |
 Caffeine Caffeine
|
 |
 |
10 mg/ml |
4°C |
 |
| 60 |
 |
|
990 |
 Caffeine Caffeine
|
 |
 |
10 mg/ml |
25°C |
 |
| 60 |
 |
|
990 |
 Caffeine Caffeine
|
 |
 |
10 mg/ml |
4°C |
 |
| 60 |
 |
|
990 |
 Calcium gluconate Calcium gluconate
|
 |
 |
mg |
25°C |
 |
| 180 |
 |
|
3017 |
 Captopril Captopril
|
 |
 |
mg/ml |
21-23°C |
 |
| 14 |
 |
|
2522 |
 Captopril Captopril
|
 |
 |
mg/ml |
21-23°C |
 |
| 28 |
 |
|
2522 |
 Captopril Captopril
|
 |
 |
mg/ml |
4°C |
 |
| 56 |
 |
|
2522 |
 Captopril Captopril
|
 |
 |
mg/ml |
4°C |
 |
| 56 |
 |
|
2522 |
 Captopril Captopril
|
 |
 |
mg/ml |
5°C |
 |
| 15 |
 |
|
3802 |
 Carboplatin Carboplatin
|
 |
 |
0,72 mg/ml |
21-22°C |
 |
| 72 |
 |
|
1520 |
 Carfilzomib Carfilzomib
|
 |
 |
2 mg/ml |
15-30°C |
 |
| 4 |
 |
|
3348 |
 Carfilzomib Carfilzomib
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3348 |
 Carfilzomib Carfilzomib
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 28 |
 |
|
3986 |
 Carfilzomib Carfilzomib
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4047 |
 Carfilzomib Carfilzomib
|
 |
 |
2 mg/ml |
25°C |
 |
| 14 |
 |
|
4047 |
 Carfilzomib Carfilzomib
|
 |
 |
0.8 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4047 |
 Carfilzomib Carfilzomib
|
 |
 |
0.8 mg/ml |
25°C |
 |
| 10 |
 |
|
4047 |
 Carfilzomib Carfilzomib
|
 |
 |
0,6 mg/ml |
2-8°C |
 |
| 28 |
 |
|
3986 |
 Carfilzomib Carfilzomib
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 10 |
 |
|
3986 |
 Carfilzomib Carfilzomib
|
 |
 |
0.6 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4047 |
 Carfilzomib Carfilzomib
|
 |
 |
0.6 mg/ml |
25°C |
 |
| 10 |
 |
|
4047 |
 Carfilzomib Carfilzomib
|
 |
 |
2 mg/ml |
15-30°C |
 |
| 4 |
 |
|
3348 |
 Carfilzomib Carfilzomib
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3348 |
 Carfilzomib Carfilzomib
|
 |
 |
2 mg/ml |
25°C |
 |
| 14 |
 |
|
3986 |
 Carfilzomib Carfilzomib
|
 |
 |
0,8 mg/ml |
2-8°C |
 |
| 28 |
 |
|
3986 |
 Carfilzomib Carfilzomib
|
 |
 |
0,8 mg/ml |
25°C |
 |
| 10 |
 |
|
3986 |
 Carfilzomib Carfilzomib
|
 |
 |
0,6 >> 1,1 mg/ml |
15-30°C |
 |
| 4 |
 |
|
3348 |
 Carfilzomib Carfilzomib
|
 |
 |
0,6 >> 1,1 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3348 |
 Carvedilol Carvedilol
|
 |
 |
mg |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
5-7 mg/ml |
25°C |
 |
| 24 |
 |
|
1519 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0.14 >> 0.47 mg/ml |
25°C |
 |
| 24 |
 |
|
1519 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0.14 >> 0.47 mg/ml |
25°C |
 |
| 24 |
 |
|
1519 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0.14 >> 0.47 mg/ml |
25°C |
 |
| 24 |
 |
|
1519 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0.14 >> 0.47 mg/ml |
25°C |
 |
| 24 |
 |
|
1519 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0.14 >> 0.47 mg/ml |
25°C |
 |
| 24 |
 |
|
1519 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0.14 >> 0.47 mg/ml |
25°C |
 |
| 24 |
 |
|
1519 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0.5 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4067 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0,2 >> 0,5 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3140 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
0,2 >> 0,5 mg/ml |
25°C |
 |
| 60 |
 |
|
3140 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
mg |
25°C |
 |
| 3 |
 |
|
3308 |
 Caspofungin acetate Caspofungin acetate
|
 |
 |
mg |
3-5°C |
 |
| 28 |
 |
|
3308 |
 Catumaxomab Catumaxomab
|
 |
 |
0,2 >> 3 µg/ml |
2-8°C |
 |
| 48 |
 |
|
2343 |
 Catumaxomab Catumaxomab
|
 |
 |
0,2 >> 3 µg/ml |
25°C |
 |
| 24 |
 |
|
2343 |
 Cefadroxil Cefadroxil
|
 |
 |
mg |
22°C |
 |
| 10 |
 |
|
2486 |
 Cefadroxil Cefadroxil
|
 |
 |
mg |
4°C |
 |
| 42 |
 |
|
2486 |
 Cefamandole nafate Cefamandole nafate
|
 |
 |
2 & 20 mg/ml |
25°C |
 |
| 72 |
 |
|
612 |
 Cefamandole nafate Cefamandole nafate
|
 |
 |
285 mg/ml |
25°C |
 |
| 72 |
 |
|
612 |
 Cefamandole nafate Cefamandole nafate
|
 |
 |
285 mg/ml |
25°C |
 |
| 72 |
 |
|
612 |
 Cefamandole nafate Cefamandole nafate
|
 |
 |
285 mg/ml |
25°C |
 |
| 72 |
 |
|
612 |
 Cefamandole nafate Cefamandole nafate
|
 |
 |
20 mg/ml |
25°C |
 |
| 72 |
 |
|
612 |
 Cefamandole nafate Cefamandole nafate
|
 |
 |
5 & 40 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 7 |
 |
|
1452 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
25°C |
 |
| 8 |
 |
|
2372 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
37°C |
 |
| 24 |
 |
|
2372 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
4°C |
 |
| 14 |
 |
|
2372 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
25°C |
 |
| 8 |
 |
|
2372 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
37°C |
 |
| 24 |
 |
|
2372 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
4°C |
 |
| 14 |
 |
|
2372 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,333 mg/ml |
25°C |
 |
| 11 |
 |
|
1979 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,333 mg/ml |
37°C |
 |
| 24 |
 |
|
1979 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,333 mg/ml |
4°C |
 |
| 20 |
 |
|
1979 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,333 mg/ml |
4°C |
 |
| 20 |
 |
|
1979 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
25°C |
 |
| 7 |
 |
|
1805 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
37°C |
 |
| 24 |
 |
|
1805 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
0,5 mg/ml |
4°C |
 |
| 30 |
 |
|
1805 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 7 |
 |
|
1452 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 7 |
 |
|
1814 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
50 mg/ml |
5°C |
 |
| 22 |
 |
|
1814 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
12,5 mg/ml |
35°C |
 |
| 12 |
 |
|
4247 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
12,5 mg/ml |
4°C |
 |
| 72 |
 |
|
4247 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
25 mg/ml |
35°C |
 |
| 12 |
 |
|
4247 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
25 mg/ml |
4°C |
 |
| 72 |
 |
|
4247 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
5 & 40 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
20 mg/ml |
25°C |
 |
| 2 |
 |
|
873 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
0,5 mg/ml |
37°C |
 |
| 4 |
 |
|
1945 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
0,5 mg/ml |
4°C |
 |
| 7 |
 |
|
1945 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
100 & 200 mg/ml |
22-24°C |
 |
| 2 |
 |
|
1046 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
200 mg/ml |
22-24°C |
 |
| 2 |
 |
|
1046 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
100 mg/ml |
22°C-24°C |
 |
| 2 |
 |
|
1046 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
20 mg/ml |
25°C |
 |
| 2 |
 |
|
1626 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
20 mg/ml |
5°C |
 |
| 21 |
 |
|
1626 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
100 mg/ml |
22°C-24°C |
 |
| 1 |
 |
|
1046 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
50 mg/ml |
25°C |
 |
| 20 |
 |
|
2042 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
50 mg/ml |
37°C |
 |
| 13 |
 |
|
2042 |
 Cefiderocol sulfate tosylate Cefiderocol sulfate tosylate
|
 |
 |
89 mg/mL |
25°C |
 |
| 1 |
 |
|
2093 |
 Cefmenoxime Cefmenoxime
|
 |
 |
2 mg/ml |
20°C |
 |
| 14 |
 |
|
587 |
 Cefmetazole sodium Cefmetazole sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 2 |
 |
|
652 |
 Cefmetazole sodium Cefmetazole sodium
|
 |
 |
20 mg/ml |
5°C |
 |
| 28 |
 |
|
652 |
 Cefmetazole sodium Cefmetazole sodium
|
 |
 |
4 & 20 mg/ml |
4°C |
 |
| 7 |
 |
|
1664 |
 Cefmetazole sodium Cefmetazole sodium
|
 |
 |
4 & 20 mg/ml |
23°C |
 |
| 48 |
 |
|
1664 |
 Cefmetazole sodium Cefmetazole sodium
|
 |
 |
4 & 20 mg/ml |
23°C |
 |
| 24 |
 |
|
1664 |
 Cefonicid sodium Cefonicid sodium
|
 |
 |
5 & 20 & 40 mg/ml |
25°C |
 |
| 24 |
 |
|
420 |
 Cefonicid sodium Cefonicid sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
420 |
 Cefonicid sodium Cefonicid sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
420 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
100 >> 300 mg/ml |
2-8°C |
 |
| 5 |
 |
|
3544 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
40 mg/ml |
25°C |
 |
| 8 |
 |
|
585 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
2 >> 50 mg/ml |
2-8°C |
 |
| 5 |
 |
|
3544 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
2 >> 50 mg/ml |
2-8°C |
 |
| 5 |
 |
|
3544 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
2 >> 50 mg/ml |
2-8°C |
 |
| 5 |
 |
|
3544 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
2 & 50 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
1452 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
10 mg/ml |
24°C |
 |
| 24 |
 |
|
593 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
0,86 mg/ml |
37°C |
 |
| 6 |
 |
|
2374 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
0,92 mg/ml |
25°C |
 |
| 24 |
 |
|
2374 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
0,80 mg/ml |
37°C |
 |
| 6 |
 |
|
2374 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
0,87 mg/ml |
25°C |
 |
| 24 |
 |
|
2374 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
1452 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 24 |
 |
|
1711 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
5 & 40 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Cefpirome sulfate Cefpirome sulfate
|
 |
 |
32 mg/ml |
25°C |
 |
| 23 |
 |
|
2042 |
 Cefpirome sulfate Cefpirome sulfate
|
 |
 |
32 mg/ml |
37°C |
 |
| 7 |
 |
|
2042 |
 Ceftaroline fosamil Ceftaroline fosamil
|
 |
 |
4>>12 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3812 |
 Ceftaroline fosamil Ceftaroline fosamil
|
 |
 |
4>>12 mg/ml |
25°C |
 |
| 6 |
 |
|
3812 |
 Ceftaroline fosamil Ceftaroline fosamil
|
 |
 |
12 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3812 |
 Ceftaroline fosamil Ceftaroline fosamil
|
 |
 |
12 mg/ml |
25°C |
 |
| 6 |
 |
|
3812 |
 Ceftazidime Ceftazidime
|
 |
 |
100 & 200 mg/ml |
21°C-23°C |
 |
| 8 |
 |
|
445 |
 Ceftazidime Ceftazidime
|
 |
 |
270 mg/ml |
4°C |
 |
| 7 |
 |
|
1857 |
 Ceftazidime Ceftazidime
|
 |
 |
40 mg/ml |
20°C |
 |
| 20 |
 |
|
1651 |
 Ceftazidime Ceftazidime
|
 |
 |
40 mg/ml |
35°C |
 |
| 20 |
 |
|
1651 |
 Ceftazidime Ceftazidime
|
 |
 |
20 mg/ml |
25°C |
 |
| 2 |
 |
|
1452 |
 Ceftazidime Ceftazidime
|
 |
 |
mg/ml |
7°C |
 |
| 7 |
 |
|
2717 |
 Ceftazidime Ceftazidime
|
 |
 |
40 mg/ml |
20°C |
 |
| 20 |
 |
|
1651 |
 Ceftazidime Ceftazidime
|
 |
 |
40 mg/ml |
20°C |
 |
| 20 |
 |
|
1651 |
 Ceftazidime Ceftazidime
|
 |
 |
20 mg/ml |
25°C |
 |
| 2 |
 |
|
1452 |
 Ceftazidime Ceftazidime
|
 |
 |
100 & 200 mg/ml |
21°C-23°C |
 |
| 8 |
 |
|
445 |
 Ceftazidime Ceftazidime
|
 |
 |
5 & 40 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Ceftazidime Ceftazidime
|
 |
 |
120 mg/ml |
27°C |
 |
| 16 |
 |
|
287 |
 Ceftazidime Ceftazidime
|
 |
 |
60 mg/ml |
27°C |
 |
| 24 |
 |
|
287 |
 Ceftazidime Ceftazidime
|
 |
 |
120 mg/ml |
25°C |
 |
| 24 |
 |
|
2042 |
 Ceftazidime Ceftazidime
|
 |
 |
120 mg/ml |
37°C |
 |
| 8 |
 |
|
2042 |
 Ceftazidime Avibactam Ceftazidime Avibactam
|
 |
 |
8/2 >> 40/10 mg/ml |
2-8°C |
 |
| 24 |
 |
|
2902 |
 Ceftazidime Avibactam Ceftazidime Avibactam
|
 |
 |
8/2 >> 40/10 mg/ml |
25°C |
 |
| 12 |
 |
|
2902 |
 Ceftazidime Avibactam Ceftazidime Avibactam
|
 |
 |
8/2 >> 40/10 mg/ml |
2-8°C |
 |
| 24 |
 |
|
2902 |
 Ceftazidime Avibactam Ceftazidime Avibactam
|
 |
 |
8/2 >> 40/10 mg/ml |
25°C |
 |
| 12 |
 |
|
2902 |
 Ceftizoxime sodium Ceftizoxime sodium
|
 |
 |
5 & 80 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
50 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 1 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
50 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 1 |
 |
|
4650 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
87.7 / 43.8 mg/ml |
2-8°C |
 |
| 4 |
 |
|
3803 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
87.7 / 43.8 mg/ml |
2-8°C |
 |
| 4 |
 |
|
3803 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.02/0.01 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.02/0.01 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.055/0.027 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.055/0.027 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.055/0.027 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.055/0.027 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.055/0.027 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.055/0.027 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.02/0.01 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.02/0.01 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.02/0.01 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.02/0.01 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.02/0.01 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.02/0.01 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.04/0.02 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.04/0.02 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.04/0.02 mg/ml |
37°C |
 |
| 12 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
0.04/0.02 mg/ml |
4°C |
 |
| 168 |
 |
|
4378 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
10 / 5 mg/ml |
2-8°C |
 |
| 7 |
 |
|
3803 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
10 / 5 mg/ml |
25°C |
 |
| 24 |
 |
|
3803 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
10 & 50 mg/ml |
15°C-32°C |
 |
| 7 |
 |
|
589 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 5 |
 |
|
1452 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
10 mg/ml |
15°C-32°C |
 |
| 7 |
 |
|
589 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
50 mg/ml |
15°C-32°C |
 |
| 24 |
 |
|
589 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
40 mg/ml |
23°C |
 |
| 3 |
 |
|
640 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
2 mg/ml |
20°C |
 |
| 14 |
 |
|
587 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
40 mg/ml |
23°C |
 |
| 1 |
 |
|
640 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
20 mg/ml |
-20°C |
 |
| 98 |
 |
|
2110 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
20 mg/ml |
2-8°C |
 |
| 14 |
 |
|
2110 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 5 |
 |
|
1452 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
5 & 40 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Cefuroxime axetil Cefuroxime axetil
|
 |
 |
mg/ml |
25°C |
 |
| 2 |
 |
|
2491 |
 Cefuroxime axetil Cefuroxime axetil
|
 |
 |
mg/ml |
25°C |
 |
| 2 |
 |
|
2491 |
 Cefuroxime axetil Cefuroxime axetil
|
 |
 |
mg/ml |
25°C |
 |
| 2 |
 |
|
2491 |
 Cefuroxime axetil Cefuroxime axetil
|
 |
 |
mg/ml |
25°C |
 |
| 2 |
 |
|
2491 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
5 & 10 mg/ml |
25°C |
 |
| 24 |
 |
|
636 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
7,5 mg/ml |
25°C |
 |
| 24 |
 |
|
1452 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
5 & 10 mg/ml |
25°C |
 |
| 24 |
 |
|
636 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
15 mg/ml |
4°C |
 |
| 31 |
 |
|
2095 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
7,5 mg/ml |
25°C |
 |
| 24 |
 |
|
1452 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 2 |
 |
|
1813 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
50 mg/ml |
5°C |
 |
| 21 |
 |
|
1813 |
 Celecoxib Celecoxib
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
4198 |
 Celecoxib Celecoxib
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
4198 |
 Cetuximab Cetuximab
|
 |
 |
5 mg/ml |
25°C |
 |
| 3 |
 |
|
4755 |
 Cetuximab Cetuximab
|
 |
 |
5 mg/ml |
25°C |
 |
| 3 |
 |
|
4755 |
 Cetuximab Cetuximab
|
 |
 |
5 mg/ml |
4°C |
 |
| 90 |
 |
|
4755 |
 Cetuximab Cetuximab
|
 |
 |
1 mg/ml |
25°C |
 |
| 3 |
 |
|
4755 |
 Cetuximab Cetuximab
|
 |
 |
1 mg/ml |
4°C |
 |
| 90 |
 |
|
4755 |
 Chloral hydrate Chloral hydrate
|
 |
 |
mg |
25°C |
 |
| 60 |
 |
|
4198 |
 Chloral hydrate Chloral hydrate
|
 |
 |
mg |
4°C |
 |
| 60 |
 |
|
4198 |
 Chlormethine hydrochloride Chlormethine hydrochloride
|
 |
 |
1 mg/ml |
21°C-23°C |
 |
| 6 |
 |
|
558 |
 Chlormethine hydrochloride Chlormethine hydrochloride
|
 |
 |
1 mg/ml |
21°C-23°C |
 |
| 6 |
 |
|
558 |
 Chlormethine hydrochloride Chlormethine hydrochloride
|
 |
 |
mg/ml |
37°C |
 |
| 40 |
 |
|
168 |
 Chlormethine hydrochloride Chlormethine hydrochloride
|
 |
 |
mg/ml |
4°C |
 |
| 84 |
 |
|
168 |
 Chlormethine hydrochloride Chlormethine hydrochloride
|
 |
 |
0,02 mg/ml |
21°C-23°C |
 |
| 3 |
 |
|
558 |
 Chlormethine hydrochloride Chlormethine hydrochloride
|
 |
 |
0,02 mg/ml |
21°C-23°C |
 |
| 4 |
 |
|
558 |
 Cholecalciferol Cholecalciferol
|
 |
 |
mg |
25°C |
 |
| 60 |
 |
|
2988 |
 Ciclosporin Ciclosporin
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
4198 |
 Ciclosporin Ciclosporin
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
4198 |
 Ciclosporin Ciclosporin
|
 |
 |
0,2 mg/ml |
23-27°C |
 |
| 14 |
 |
|
3229 |
 Ciclosporin Ciclosporin
|
 |
 |
2,5 mg/ml |
23-27°C |
 |
| 14 |
 |
|
3229 |
 Ciclosporin Ciclosporin
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 7 |
 |
|
3476 |
 Ciclosporin Ciclosporin
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 14 |
 |
|
3476 |
 Ciprofloxacin hydrochloride Ciprofloxacin hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
4198 |
 Ciprofloxacin hydrochloride Ciprofloxacin hydrochloride
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
4198 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
? mg/ml |
25°C |
 |
| 90 |
 |
|
666 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
0,025 mg/ml |
25°C |
 |
| 7 |
 |
|
4263 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
0,025 mg/ml |
37°C |
 |
| 48 |
 |
|
4263 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
0,025 mg/ml |
25°C |
 |
| 7 |
 |
|
4263 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
0,025 mg/ml |
37°C |
 |
| 48 |
 |
|
4263 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
5 & 20 mg/ml |
25°C |
 |
| 30 |
 |
|
604 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
0,1 mg/ml |
23-27°C |
 |
| 48 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
0,1 mg/ml |
4-6°C |
 |
| 7 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
0,1 mg/ml |
23-27°C |
 |
| 24 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
0,1 mg/ml |
4-6°C |
 |
| 7 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
0,1 mg/ml |
23-27°C |
 |
| 2 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
0,1 mg/ml |
4-6°C |
 |
| 7 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
0,1 mg/ml |
23-27°C |
 |
| 2 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
0,1 mg/ml |
4-6°C |
 |
| 2 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
2 mg/ml |
23-27°C |
 |
| 7 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
2 mg/ml |
4-6°C |
 |
| 7 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
5 mg/ml |
23-27°C |
 |
| 7 |
 |
|
4358 |
 Cisatracurium besylate Cisatracurium besylate
|
 |
 |
5 mg/ml |
4-6°C |
 |
| 7 |
 |
|
4358 |
 Cladribine Cladribine
|
 |
 |
? mg/ml |
25°C |
 |
| 24 |
 |
|
3579 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
6 , 9 & 12 mg/ml |
25°C |
 |
| 16 |
 |
|
566 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9 mg/ml |
25°C |
 |
| 48 |
 |
|
565 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
6 , 9 & 12 mg/ml |
25°C |
 |
| 16 |
 |
|
566 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
6 & 12 mg/ml |
25°C |
 |
| 22 |
 |
|
567 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
2 & 12 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9,5 mg/ml |
2-8°C |
 |
| 49 |
 |
|
4622 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9,5 mg/ml |
25°C |
 |
| 24 |
 |
|
4622 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9,5 mg/ml |
2-8°C |
 |
| 47 |
 |
|
4619 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9,5 mg/ml |
25°C |
 |
| 24 |
 |
|
4619 |
 Clofarabine Clofarabine
|
 |
 |
0,2 >> 0,65 mg/ml |
2-8°C |
 |
| 3 |
 |
|
2272 |
 Clofarabine Clofarabine
|
 |
 |
0,2 >> 0,65 mg/ml |
25°C |
 |
| 3 |
 |
|
2272 |
 Clomipramine hydrochloride Clomipramine hydrochloride
|
 |
 |
? mg/ml |
25°C |
 |
| 4 |
 |
|
54 |
 Clomipramine hydrochloride Clomipramine hydrochloride
|
 |
 |
? mg/ml |
25°C |
 |
| 4 |
 |
|
54 |
 Clonazepam Clonazepam
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 10 |
 |
|
1246 |
 Clonazepam Clonazepam
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 10 |
 |
|
1246 |
 Clonidine hydrochloride Clonidine hydrochloride
|
 |
 |
2 mg/ml |
40°C |
 |
| 180 |
 |
|
2317 |
 Clonidine hydrochloride Clonidine hydrochloride
|
 |
 |
mg |
25°C |
 |
| 91 |
 |
|
3649 |
 Clonidine hydrochloride Clonidine hydrochloride
|
 |
 |
mg |
4°C |
 |
| 91 |
 |
|
3649 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
250 mg/ml |
25°C |
 |
| 1 |
 |
|
4244 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
250 mg/ml |
4°C |
 |
| 14 |
 |
|
4244 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
5 >> 50 mg/ml |
23°C |
 |
| 1 |
 |
|
1278 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
5 >> 40 mg/ml |
23°C |
 |
| 4 |
 |
|
1278 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
50 mg/ml |
23°C |
 |
| 2 |
 |
|
1278 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
100 mg/ml |
25°C |
 |
| 1 |
 |
|
4244 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
100 mg/ml |
4°C |
 |
| 14 |
 |
|
4244 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 1 |
 |
|
4244 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
50 mg/ml |
4°C |
 |
| 14 |
 |
|
4244 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
0,64 / 3,2 mg/ml |
23°C-25°C |
 |
| 48 |
 |
|
442 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
1,6 / 8 mg/ml |
23°C-25°C |
 |
| 1 |
 |
|
442 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
0,6/3,2 mg/ml |
23-25°C |
 |
| 24 |
 |
|
1197 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
0,8 / 4 mg/ml |
23°C-25°C |
 |
| 14 |
 |
|
442 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
1,07 / 5,33 mg/ml |
23°C-25°C |
 |
| 2 |
 |
|
442 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
1,6/8 mg/ml |
23-25°C |
 |
| 24 |
 |
|
1197 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
0,6/3,2 mg/ml |
23-25°C |
 |
| 24 |
 |
|
1197 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
0,8 / 4 mg/ml |
23°C-25°C |
 |
| 24 |
 |
|
442 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
1,07 / 5,33 mg/ml |
23°C-25°C |
 |
| 4 |
 |
|
442 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
1,6/8 mg/ml |
23-25°C |
 |
| 24 |
 |
|
1197 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
0,6/3,2 mg/ml |
23-25°C |
 |
| 12 |
 |
|
1197 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
1,6/8 mg/ml |
23-25°C |
 |
| 24 |
 |
|
1197 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
0,6/3,2 mg/ml |
23-25°C |
 |
| 24 |
 |
|
1197 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
1,6/8 mg/ml |
23-25°C |
 |
| 24 |
 |
|
1197 |
 Cocaine hydrochloride Cocaine hydrochloride
|
 |
 |
mg/ml |
2-8°C |
 |
| 14 |
 |
|
2808 |
 Cocaine hydrochloride Cocaine hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 48 |
 |
|
2808 |
 Colistin mesilate sodium Colistin mesilate sodium
|
 |
 |
75 mg/ml |
21°C |
 |
| 24 |
 |
|
3360 |
 Colistin mesilate sodium Colistin mesilate sodium
|
 |
 |
75 mg/ml |
4°C |
 |
| 24 |
 |
|
3360 |
 Colistin mesilate sodium Colistin mesilate sodium
|
 |
 |
0,8 mg/ml |
4°C |
 |
| 3 |
 |
|
2783 |
 Colistin mesilate sodium Colistin mesilate sodium
|
 |
 |
mg/ml |
2-8°C |
 |
| 48 |
 |
|
3334 |
 Colistin mesilate sodium Colistin mesilate sodium
|
 |
 |
mg/ml |
2-8°C |
 |
| 48 |
 |
|
3334 |
 Crisantaspase Crisantaspase
|
 |
 |
5000 & 10000 UI |
<25°C |
 |
| 8 |
 |
|
4480 |
 Crisantaspase Crisantaspase
|
 |
 |
5000 & 10000 UI |
<25°C |
 |
| 8 |
 |
|
4480 |
 Cyclophosphamide Cyclophosphamide
|
 |
 |
20 mg/ml |
24-27°C |
 |
| 4 |
 |
|
1728 |
 Cyclophosphamide Cyclophosphamide
|
 |
 |
0,4 mg/ml |
21-25°C |
 |
| 6 |
 |
|
1728 |
 Cyclophosphamide Cyclophosphamide
|
 |
 |
mg |
? |
 |
| 70 |
 |
|
2000 |
 Cytarabine Cytarabine
|
 |
 |
3 mg/ml |
23°C |
 |
| 48 |
 |
|
31 |
 Cytarabine Cytarabine
|
 |
 |
0,144 mg/ml |
21°C-22°C |
 |
| 72 |
 |
|
1520 |
 Cytarabine Cytarabine
|
 |
 |
0,144 mg/ml |
4°C |
 |
| 72 |
 |
|
1520 |
 Cytarabine Cytarabine
|
 |
 |
3 mg/ml |
23°C |
 |
| 48 |
 |
|
31 |
 Cytarabine Cytarabine
|
 |
 |
20 & 50 mg/ml |
22°C |
 |
| 14 |
 |
|
850 |
 Dactinomycin Dactinomycin
|
 |
 |
10 µg/ml |
20-25°C |
 |
| 10 |
 |
|
4153 |
 Dactinomycin Dactinomycin
|
 |
 |
10 µg/ml |
20-25°C |
 |
| 10 |
 |
|
4153 |
 Dactinomycin Dactinomycin
|
 |
 |
10 µg/ml |
20-25°C |
 |
| 10 |
 |
|
4153 |
 Dactinomycin Dactinomycin
|
 |
 |
10 µg/ml |
20-25°C |
 |
| 10 |
 |
|
4153 |
 Dalbavancin Dalbavancin
|
 |
 |
1 >> 5 mg/ml |
2-8°C |
 |
| 48 |
 |
|
3752 |
 Dalbavancin Dalbavancin
|
 |
 |
1 >> 5 mg/ml |
20-25°C |
 |
| 48 |
 |
|
3752 |
 Dalteparin sodium Dalteparin sodium
|
 |
 |
2500 UI/ml |
4°C |
 |
| 28 |
 |
|
2268 |
 Dalteparin sodium Dalteparin sodium
|
 |
 |
10000 UI/ml |
25°C |
 |
| 30 |
 |
|
1873 |
 Dalteparin sodium Dalteparin sodium
|
 |
 |
25000 UI/ml |
4°C |
 |
| 30 |
 |
|
1873 |
 Daptomycin Daptomycin
|
 |
 |
50 mg/ml |
2-8°C |
 |
| 48 |
 |
|
2271 |
 Daptomycin Daptomycin
|
 |
 |
50 mg/ml |
25°C |
 |
| 12 |
 |
|
2271 |
 Daptomycin Daptomycin
|
 |
 |
50 mg/ml |
2-8°C |
 |
| 48 |
 |
|
2271 |
 Daptomycin Daptomycin
|
 |
 |
50 mg/ml |
25°C |
 |
| 12 |
 |
|
2271 |
 Daptomycin Daptomycin
|
 |
 |
0.02 mg/ml |
25°C |
 |
| 24 |
 |
|
3439 |
 Daptomycin Daptomycin
|
 |
 |
0.02 mg/ml |
37°C |
 |
| 6 |
 |
|
3439 |
 Daptomycin Daptomycin
|
 |
 |
0.02 mg/ml |
25°C |
 |
| 24 |
 |
|
3439 |
 Daptomycin Daptomycin
|
 |
 |
0.02 mg/ml |
37°C |
 |
| 6 |
 |
|
3439 |
 Daptomycin Daptomycin
|
 |
 |
? mg/ml |
2-8°C |
 |
| 24 |
 |
|
2271 |
 Daptomycin Daptomycin
|
 |
 |
? mg/ml |
25°C |
 |
| 12 |
 |
|
2271 |
 Daratumumab Daratumumab
|
 |
 |
120 mg/mL |
15-25°C |
 |
| 24 |
 |
|
4785 |
 Daratumumab Daratumumab
|
 |
 |
120 mg/mL |
2-8°C |
 |
| 24 |
 |
|
4785 |
 Daunorubicin hydrochloride Daunorubicin hydrochloride
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 43 |
 |
|
686 |
 Daunorubicin hydrochloride liposome Daunorubicin hydrochloride liposome
|
 |
 |
0,2 >> 1 mg/ml |
25°C |
 |
| 6 |
 |
|
3577 |
 Daunorubicin/cytarabine liposomale Daunorubicin/cytarabine liposomale
|
 |
 |
2.2 / 5 mg/mL |
2-8°C |
 |
| 4 |
 |
|
4355 |
 Daunorubicin/cytarabine liposomale Daunorubicin/cytarabine liposomale
|
 |
 |
2.2 / 5 mg/mL |
2-8°C |
 |
| 4 |
 |
|
4355 |
 Daunorubicin/cytarabine liposomale Daunorubicin/cytarabine liposomale
|
 |
 |
0.087 / 0.195 mg/mL |
2-8°C |
 |
| 4 |
 |
|
4355 |
 Daunorubicin/cytarabine liposomale Daunorubicin/cytarabine liposomale
|
 |
 |
0.176 / 0.4 mg/mL |
2-8°C |
 |
| 4 |
 |
|
4355 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
100 mg/ml |
25°C |
 |
| 24 |
 |
|
3671 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
0,35 mg/ml |
25°C |
 |
| 7 |
 |
|
2156 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
0,35 mg/ml |
37°C |
 |
| 48 |
 |
|
2156 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
0,35 mg/ml |
4°C |
 |
| 15 |
 |
|
2156 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
0,35 mg/ml |
25°C |
 |
| 7 |
 |
|
2156 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
0,35 mg/ml |
37°C |
 |
| 48 |
 |
|
2156 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
0,35 mg/ml |
4°C |
 |
| 15 |
 |
|
2156 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
250 mg/ml |
30°C |
 |
| 14 |
 |
|
5 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
5 mg/ml |
25°C |
 |
| 12 |
 |
|
604 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
? mg/ml |
25°C |
 |
| 24 |
 |
|
3671 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
? mg/ml |
25°C |
 |
| 36 |
 |
|
3671 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
? mg/ml |
25°C |
 |
| 24 |
 |
|
3671 |
 Defibrotide Defibrotide
|
 |
 |
4 >> 20 mg/ml |
15-25°C |
 |
| 72 |
 |
|
3701 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
0,08 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4602 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
0,08 mg/ml |
20-23°C |
 |
| 14 |
 |
|
4602 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
0,4 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4602 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
0,4 mg/ml |
20-23°C |
 |
| 14 |
 |
|
4602 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
0,1 & 1 mg/ml |
25°C |
 |
| 22 |
 |
|
1701 |
 Dexmedetomidine Dexmedetomidine
|
 |
 |
4 µg/ml |
20-25°C |
 |
| 14 |
 |
|
4029 |
 Dexmedetomidine Dexmedetomidine
|
 |
 |
4 µg/ml |
3-4°C |
 |
| 14 |
 |
|
4029 |
 Dexmedetomidine Dexmedetomidine
|
 |
 |
4 >> 20 µg/ml |
21-25°C |
 |
| 48 |
 |
|
3433 |
 Dexmedetomidine Dexmedetomidine
|
 |
 |
4 µg/ml |
20-25°C |
 |
| 14 |
 |
|
4029 |
 Dexmedetomidine Dexmedetomidine
|
 |
 |
4 µg/ml |
3-4°C |
 |
| 14 |
 |
|
4029 |
 Dexmedetomidine Dexmedetomidine
|
 |
 |
4 µg/ml |
20-25°C |
 |
| 2 |
 |
|
3299 |
 Dexmedetomidine Dexmedetomidine
|
 |
 |
4 µg/ml |
5°C |
 |
| 14 |
 |
|
3299 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
3 & 4 mg/ml |
25°C |
 |
| 8 |
 |
|
2224 |
 Diamorphine hydrochloride Diamorphine hydrochloride
|
 |
 |
1 mg/ml |
23°C-25°C |
 |
| 7 |
 |
|
339 |
 Diamorphine hydrochloride Diamorphine hydrochloride
|
 |
 |
20 mg/ml |
23-25°C |
 |
| 12 |
 |
|
339 |
 Diamorphine hydrochloride Diamorphine hydrochloride
|
 |
 |
1 & 20 mg/ml |
23-25°C |
 |
| 15 |
 |
|
339 |
 Diamorphine hydrochloride Diamorphine hydrochloride
|
 |
 |
1 & 20 mg/ml |
23-25°C |
 |
| 7 |
 |
|
339 |
 Diazepam Diazepam
|
 |
 |
0,04 mg/ml |
25°C |
 |
| 48 |
 |
|
55 |
 Diazepam Diazepam
|
 |
 |
0,04 mg/ml |
25°C |
 |
| 48 |
 |
|
55 |
 Dichloroacetate sodium Dichloroacetate sodium
|
 |
 |
mg/mL |
25°C |
 |
| 90 |
 |
|
4283 |
 Dichloroacetate sodium Dichloroacetate sodium
|
 |
 |
mg/mL |
4°C |
 |
| 90 |
 |
|
4283 |
 Diclofenac Diclofenac
|
 |
 |
mg |
25°C |
 |
| 120 |
 |
|
2567 |
 Dinoprostone Dinoprostone
|
 |
 |
5 µg/ml |
25°C |
 |
| 24 |
 |
|
1121 |
 Diphenhydramine hydrochloride Diphenhydramine hydrochloride
|
 |
 |
0,2 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4185 |
 Diphenhydramine hydrochloride Diphenhydramine hydrochloride
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4185 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
628 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 12 |
 |
|
4141 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
10 mg/ml |
35°C |
 |
| 12 |
 |
|
4141 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
10 mg/ml |
4°C |
 |
| 7 |
 |
|
4141 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
628 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
628 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
0,0025 mg/ml |
26°C |
 |
| 4 |
 |
|
2371 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
0,0025 mg/ml |
4°C |
 |
| 24 |
 |
|
2371 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
0,005 mg/ml |
26°C |
 |
| 24 |
 |
|
2371 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
0,005 mg/ml |
37°C |
 |
| 24 |
 |
|
2371 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
0,005 mg/ml |
4°C |
 |
| 24 |
 |
|
2371 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
0,0075 mg/ml |
26°C |
 |
| 24 |
 |
|
2371 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
0,0075 mg/ml |
37°C |
 |
| 24 |
 |
|
2371 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
0,0075 mg/ml |
4°C |
 |
| 24 |
 |
|
2371 |
 Docetaxel Docetaxel
|
 |
 |
< 0,74 mg/ml |
2-8°C |
 |
| 48 |
 |
|
3130 |
 Docetaxel Docetaxel
|
 |
 |
< 0,74 mg/ml |
25°C |
 |
| 6 |
 |
|
3130 |
 Docetaxel Docetaxel
|
 |
 |
0,24 >> 1 mg/ml |
20°C |
 |
| 28 |
 |
|
3215 |
 Docetaxel Docetaxel
|
 |
 |
0,24 >> 1 mg/ml |
5°C |
 |
| 28 |
 |
|
3215 |
 Dolasetron mesylate Dolasetron mesylate
|
 |
 |
10 mg/ml |
23°C |
 |
| 31 |
 |
|
1807 |
 Dolasetron mesylate Dolasetron mesylate
|
 |
 |
10 mg/ml |
4°C |
 |
| 31 |
 |
|
1807 |
 Dolasetron mesylate Dolasetron mesylate
|
 |
 |
20 mg/ml |
25°C |
 |
| 240 |
 |
|
1986 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
10 mg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
2 mg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
30 mg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
10 mg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
2 mg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
30 mg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
0,4 >> 3,2 mg/ml |
25°C |
 |
| 48 |
 |
|
3638 |
 Doripenem Doripenem
|
 |
 |
10 mg/ml |
25°C |
 |
| 24 |
 |
|
3112 |
 Doripenem Doripenem
|
 |
 |
10 mg/ml |
37°C |
 |
| 12 |
 |
|
3112 |
 Doripenem Doripenem
|
 |
 |
50 mg/ml |
25°C |
 |
| 1 |
 |
|
2332 |
 Doripenem Doripenem
|
 |
 |
50 mg/ml |
25°C |
 |
| 1 |
 |
|
2332 |
 Doripenem Doripenem
|
 |
 |
10 mg/ml |
4°C |
 |
| 7 |
 |
|
3145 |
 Doripenem Doripenem
|
 |
 |
5 mg/ml |
4°C |
 |
| 10 |
 |
|
3145 |
 Doripenem Doripenem
|
 |
 |
5 et 10 mg/ml |
25°C |
 |
| 24 |
 |
|
3145 |
 Doripenem Doripenem
|
 |
 |
5 et 10 mg/ml |
25°C |
 |
| 16 |
 |
|
3145 |
 Doripenem Doripenem
|
 |
 |
10 mg/ml |
4°C |
 |
| 10 |
 |
|
3145 |
 Doripenem Doripenem
|
 |
 |
5 mg/ml |
4°C |
 |
| 10 |
 |
|
3145 |
 Doripenem Doripenem
|
 |
 |
5 et 10 mg/ml |
25°C |
 |
| 24 |
 |
|
3145 |
 Doripenem Doripenem
|
 |
 |
5 et 10 mg/ml |
25°C |
 |
| 16 |
 |
|
3145 |
 Doripenem Doripenem
|
 |
 |
4.5 mg/ml |
2-8°C |
 |
| 72 |
 |
|
2332 |
 Doripenem Doripenem
|
 |
 |
4.5 mg/ml |
25°C |
 |
| 12 |
 |
|
2332 |
 Doripenem Doripenem
|
 |
 |
4.5 mg/ml |
25°C |
 |
| 4 |
 |
|
2332 |
 Doxorubicin hydrochloride Doxorubicin hydrochloride
|
 |
 |
0,1 mg/ml |
22°C |
 |
| 8 |
 |
|
1897 |
 Doxorubicin hydrochloride Doxorubicin hydrochloride
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 24 |
 |
|
686 |
 Doxorubicin hydrochloride liposome Doxorubicin hydrochloride liposome
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 5 |
 |
|
3143 |
 Doxorubicin hydrochloride liposome Doxorubicin hydrochloride liposome
|
 |
 |
2 mg/ml |
25°C |
 |
| 8 |
 |
|
3143 |
 Doxorubicin hydrochloride liposome Doxorubicin hydrochloride liposome
|
 |
 |
0.4 >> 1.2 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3143 |
 Doxycycline hyclate Doxycycline hyclate
|
 |
 |
0,8 & 1 mg/ml |
23°C |
 |
| 96 |
 |
|
1664 |
 Doxycycline hyclate Doxycycline hyclate
|
 |
 |
0,8 & 1 mg/ml |
4°C |
 |
| 7 |
 |
|
1664 |
 Durvalumab Durvalumab
|
 |
 |
1 >> 15 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4608 |
 Durvalumab Durvalumab
|
 |
 |
1 >> 15 mg/ml |
25°C |
 |
| 24 |
 |
|
4608 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 90 |
 |
|
4782 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
28-32°C |
 |
| 72 |
 |
|
4782 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 90 |
 |
|
4782 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
28-32°C |
 |
| 72 |
 |
|
4782 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 90 |
 |
|
4782 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
28-32°C |
 |
| 72 |
 |
|
4782 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 90 |
 |
|
4782 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
28-32°C |
 |
| 72 |
 |
|
4782 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 24 |
 |
|
2348 |
 Eculizumab Eculizumab
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 24 |
 |
|
2348 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
2-8°C |
 |
| 50 |
 |
|
4097 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
23-27°C |
 |
| 90 |
 |
|
3921 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
23-27°C |
 |
| 90 |
 |
|
3921 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
23-27°C |
 |
| 50 |
 |
|
4097 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
3-7°C |
 |
| 90 |
 |
|
3921 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
3-7°C |
 |
| 90 |
 |
|
3921 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
25°C |
 |
| 91 |
 |
|
2425 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
4°C |
 |
| 91 |
 |
|
2425 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
4°C |
 |
| 91 |
 |
|
2425 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
23-27°C |
 |
| 90 |
 |
|
3921 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
23-27°C |
 |
| 90 |
 |
|
3921 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
3-7°C |
 |
| 90 |
 |
|
3921 |
 Enalapril maleate Enalapril maleate
|
 |
 |
mg/ml |
3-7°C |
 |
| 90 |
 |
|
3921 |
 Enalaprilate Enalaprilate
|
 |
 |
12 mg/ml |
25°C |
 |
| 24 |
 |
|
963 |
 Enoxaparin sodium Enoxaparin sodium
|
 |
 |
20 mg/ml |
22°C-26°C |
 |
| 28 |
 |
|
1893 |
 Enoxaparin sodium Enoxaparin sodium
|
 |
 |
20 mg/ml |
22°C-26°C |
 |
| 14 |
 |
|
1893 |
 Enoxaparin sodium Enoxaparin sodium
|
 |
 |
8 mg/ml |
2-6°C |
 |
| 14 |
 |
|
4413 |
 Ephedrine hydrochloride Ephedrine hydrochloride
|
 |
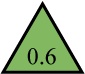 |
10 mg/ml |
25°C |
 |
| 365 |
 |
|
2035 |
 Ephedrine hydrochloride Ephedrine hydrochloride
|
 |
 |
10 mg/ml |
40°C |
 |
| 365 |
 |
|
2035 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
20 µg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
300 µg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
900 µg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
10 µg/ml |
25°C |
 |
| 21 |
 |
|
4393 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
10 µg/ml |
4°C |
 |
| 56 |
 |
|
4393 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
20 µg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
300 µg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
900 µg/ml |
23°C |
 |
| 84 |
 |
|
3712 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
1 & 2 mg/ml |
23°C |
 |
| 150 |
 |
|
683 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 20 |
 |
|
686 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
0,2 mg/ml |
2-8°C |
 |
| 84 |
 |
|
3632 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
0,2 mg/ml |
25 °C |
 |
| 14 |
 |
|
3632 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 84 |
 |
|
3632 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
1 mg/ml |
25 °C |
 |
| 14 |
 |
|
3632 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
0,5 mg/ml |
20°C |
 |
| 28 |
 |
|
1317 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
1 mg/ml |
8°C |
 |
| 84 |
 |
|
1823 |
 Epoetin alfa Epoetin alfa
|
 |
 |
100 UI/ml |
25°C |
 |
| 3 |
 |
|
798 |
 Epoetin alfa Epoetin alfa
|
 |
 |
20000 UI/ml |
2-8°C |
 |
| 42 |
 |
|
1804 |
 Epoetin alfa Epoetin alfa
|
 |
 |
5000 UI/ml |
30°C |
 |
| 84 |
 |
|
304 |
 Eravacycline Eravacycline
|
 |
 |
10 mg/ml |
25°C |
 |
| 1 |
 |
|
4709 |
 Eravacycline Eravacycline
|
 |
 |
10 mg/ml |
25°C |
 |
| 1 |
 |
|
4709 |
 Eravacycline Eravacycline
|
 |
 |
0,2 >>0,6 mg/ml |
2-8°C |
 |
| 10 |
 |
|
4709 |
 Eravacycline Eravacycline
|
 |
 |
0,2 >>0,6 mg/ml |
25°C |
 |
| 24 |
 |
|
4709 |
 Eribulin mesylate Eribulin mesylate
|
 |
 |
0,018 >> 0,18 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3250 |
 Ertapenem Ertapenem
|
 |
 |
10 & 20 mg/ml |
25°C |
 |
| 6 |
 |
|
1906 |
 Ertapenem Ertapenem
|
 |
 |
10 & 20 mg/ml |
4°C |
 |
| 5 |
 |
|
1906 |
 Ertapenem Ertapenem
|
 |
 |
10 & 20 mg/ml |
4°C |
 |
| 3 |
 |
|
1906 |
 Ertapenem Ertapenem
|
 |
 |
100 mg/ml |
-20°C |
 |
| 28 |
 |
|
3717 |
 Ertapenem Ertapenem
|
 |
 |
100 mg/ml |
4°C |
 |
| 24 |
 |
|
3717 |
 Ertapenem Ertapenem
|
 |
 |
10 mg/ml |
4-7°C |
 |
| 3 |
 |
|
3208 |
 Ertapenem Ertapenem
|
 |
 |
20 mg/ml |
2-8°C |
 |
| 24 |
 |
|
1912 |
 Ertapenem Ertapenem
|
 |
 |
20 mg/ml |
25°C |
 |
| 6 |
 |
|
1912 |
 Erythromycin lactobionate Erythromycin lactobionate
|
 |
 |
50 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3674 |
 Esketamine Esketamine
|
 |
 |
1 & 2 mg/ml |
25°C |
 |
| 48 |
 |
|
4700 |
 Esketamine Esketamine
|
 |
 |
1 & 2 mg/ml |
25°C |
 |
| 48 |
 |
|
4700 |
 Eslicarbazepine acetate Eslicarbazepine acetate
|
 |
 |
mg/ml |
20-25°C |
 |
| 48 |
 |
|
4048 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
4 & 8 mg/ml |
< 30°C |
 |
| 12 |
 |
|
2284 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0,4 mg/ml |
23°C |
 |
| 48 |
 |
|
2270 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0,4 mg/ml |
4°C |
 |
| 5 |
 |
|
2270 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0,8 mg/ml |
23°C |
 |
| 48 |
 |
|
2270 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0,8 mg/ml |
4°C |
 |
| 5 |
 |
|
2270 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0,4 mg/ml |
23°C |
 |
| 48 |
 |
|
2270 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0,4 mg/ml |
4°C |
 |
| 5 |
 |
|
2270 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0,8 mg/ml |
23°C |
 |
| 48 |
 |
|
2270 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0,8 mg/ml |
4°C |
 |
| 5 |
 |
|
2270 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0.4 & 0.8 mg/ml |
< 30°C |
 |
| 12 |
 |
|
2284 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0.4 & 0.8 mg/ml |
< 30°C |
 |
| 6 |
 |
|
2284 |
 Esomeprazole sodium Esomeprazole sodium
|
 |
 |
0.4 & 0.8 mg/ml |
< 30°C |
 |
| 12 |
 |
|
2284 |
 Ethacrynate sodium Ethacrynate sodium
|
 |
 |
1 mg/ml |
25°C |
 |
| 14 |
 |
|
1716 |
 Ethanol Ethanol
|
 |
 |
70 % |
23-25°C |
 |
| 14 |
 |
|
2221 |
 Ethanol Ethanol
|
 |
 |
70 % |
23-25°C |
 |
| 14 |
 |
|
2221 |
 Etoposide Etoposide
|
 |
 |
0,2 mg/ml |
? |
 |
| 7 |
 |
|
504 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
24°C |
 |
| 24 |
 |
|
161 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
4°C |
 |
| 24 |
 |
|
161 |
 Etoposide Etoposide
|
 |
 |
0.25 mg/ml |
8-12°C |
 |
| 21 |
 |
|
2167 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
24°C |
 |
| 24 |
 |
|
161 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
4°C |
 |
| 24 |
 |
|
161 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
0,1 & 10 mg/ml |
23°C |
 |
| 31 |
 |
|
1411 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
0,1 & 10 mg/ml |
32°C |
 |
| 7 |
 |
|
1411 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
10 & 20 mg/ml |
23°C |
 |
| 31 |
 |
|
1411 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
10 & 20 mg/ml |
32°C |
 |
| 7 |
 |
|
1411 |
 Famotidine Famotidine
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 15 |
 |
|
693 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
10 & 50 µg/ml |
23°C |
 |
| 90 |
 |
|
4228 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
10 & 50 µg/ml |
4°C |
 |
| 90 |
 |
|
4228 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
20 µg/ml |
23°C |
 |
| 30 |
 |
|
222 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
20 µg/ml |
3°C |
 |
| 30 |
 |
|
222 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
50 µg/ml |
25°C |
 |
| 14 |
 |
|
2118 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
10 & 50 µg/ml |
23°C |
 |
| 90 |
 |
|
4228 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
10 & 50 µg/ml |
4°C |
 |
| 90 |
 |
|
4228 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
10 µg/ml |
25°C |
 |
| 100 |
 |
|
4303 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
13 & 34 µg/ml |
22°C |
 |
| 7 |
 |
|
929 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
13 & 34 µg/ml |
38°C |
 |
| 7 |
 |
|
929 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
20 & 40 µg/ml |
23°C |
 |
| 90 |
 |
|
4223 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
50 µg/ml |
23°C |
 |
| 90 |
 |
|
4223 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
50 µg/ml |
25°C |
 |
| 100 |
 |
|
4303 |
 Ferric carboxymaltose Ferric carboxymaltose
|
 |
 |
1 mg/ml |
30°C |
 |
| 72 |
 |
|
2292 |
 Flecainide acetate Flecainide acetate
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
4198 |
 Flecainide acetate Flecainide acetate
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
4198 |
 Floxuridine Floxuridine
|
 |
 |
1 mg/ml |
30°C |
 |
| 21 |
 |
|
5 |
 Floxuridine Floxuridine
|
 |
 |
50 mg/ml |
30°C |
 |
| 21 |
 |
|
5 |
 Flucloxacillin sodium Flucloxacillin sodium
|
 |
 |
120 mg/ml |
2-8°C |
 |
| 6 |
 |
|
3189 |
 Flucloxacillin sodium Flucloxacillin sodium
|
 |
 |
50 mg/ml |
2-8°C |
 |
| 6 |
 |
|
3189 |
 Flucloxacillin sodium Flucloxacillin sodium
|
 |
 |
10 & 50 mg/ml |
2-8°C |
 |
| 13 |
 |
|
4218 |
 Flucloxacillin sodium Flucloxacillin sodium
|
 |
 |
10 & 50 mg/ml |
32°C |
 |
| 24 |
 |
|
4218 |
 Flucloxacillin sodium Flucloxacillin sodium
|
 |
 |
10 & 50 mg/ml |
2-8°C |
 |
| 13 |
 |
|
4218 |
 Flucloxacillin sodium Flucloxacillin sodium
|
 |
 |
10 & 50 mg/ml |
32°C |
 |
| 24 |
 |
|
4218 |
 Fludrocortisone acetate Fludrocortisone acetate
|
 |
 |
µg |
25°C |
 |
| 180 |
 |
|
4646 |
 Fludrocortisone acetate Fludrocortisone acetate
|
 |
 |
µg |
25°C |
 |
| 180 |
 |
|
4646 |
 Fludrocortisone acetate Fludrocortisone acetate
|
 |
 |
µg |
25°C |
 |
| 180 |
 |
|
4646 |
 Flumazenil Flumazenil
|
 |
 |
mg/ml |
2-8°C |
 |
| 24 |
 |
|
3565 |
 Flumazenil Flumazenil
|
 |
 |
mg/ml |
25°C |
 |
| 24 |
 |
|
3565 |
 Flumazenil Flumazenil
|
 |
 |
mg/ml |
2-8°C |
 |
| 24 |
 |
|
3565 |
 Flumazenil Flumazenil
|
 |
 |
mg/ml |
25°C |
 |
| 24 |
 |
|
3565 |
 Fluorouracil Fluorouracil
|
 |
 |
1,44 mg/ml |
21-22°C |
 |
| 48 |
 |
|
1520 |
 Fluorouracil Fluorouracil
|
 |
 |
1,44 mg/ml |
4°C |
 |
| 48 |
 |
|
1520 |
 Fluorouracil Fluorouracil
|
 |
 |
7 mg/ml |
21-25°C |
 |
| 17 |
 |
|
4735 |
 Fluorouracil Fluorouracil
|
 |
 |
8 mg/ml |
21-25°C |
 |
| 24 |
 |
|
4735 |
 Fluorouracil Fluorouracil
|
 |
 |
50 mg/ml |
30°C |
 |
| 21 |
 |
|
5 |
 Fluorouracil Fluorouracil
|
 |
 |
5 & 30 mg/ml |
25°C |
 |
| 60 |
 |
|
4759 |
 Fluorouracil Fluorouracil
|
 |
 |
5 & 30 mg/ml |
33°C |
 |
| 7 |
 |
|
4759 |
 Fluorouracil Fluorouracil
|
 |
 |
50 mg/ml |
25°C |
 |
| 60 |
 |
|
4759 |
 Fluorouracil Fluorouracil
|
 |
 |
50 mg/ml |
33°C |
 |
| 7 |
 |
|
4759 |
 Fluorouracil Fluorouracil
|
 |
 |
25 mg/ml |
25°C |
 |
| 21 |
 |
|
1817 |
 Fluorouracil Fluorouracil
|
 |
 |
25 mg/ml |
31°C |
 |
| 21 |
 |
|
1817 |
 Fluorouracil Fluorouracil
|
 |
 |
25 mg/ml |
4°C |
 |
| 14 |
 |
|
1817 |
 Fluorouracil Fluorouracil
|
 |
 |
50 mg/ml |
25°C |
 |
| 21 |
 |
|
1817 |
 Fluorouracil Fluorouracil
|
 |
 |
50 mg/ml |
31°C |
 |
| 21 |
 |
|
1817 |
 Folinate sodium Folinate sodium
|
 |
 |
3.2 mg/ml |
4°C |
 |
| 30 |
 |
|
2204 |
 Foscarnet sodium Foscarnet sodium
|
 |
 |
12 mg/ml |
25°C |
 |
| 35 |
 |
|
75 |
 Foscarnet sodium Foscarnet sodium
|
 |
 |
2 & 20 mg/ml |
25°C |
 |
| 4 |
 |
|
604 |
 Fosfomycin Fosfomycin
|
 |
 |
16 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3663 |
 Fosfomycin Fosfomycin
|
 |
 |
16 mg/ml |
25°C |
 |
| 24 |
 |
|
3663 |
 Fosphenytoin sodium Fosphenytoin sodium
|
 |
 |
1 & 8 & 20 mg/ml |
25°C |
 |
| 30 |
 |
|
800 |
 Fosphenytoin sodium Fosphenytoin sodium
|
 |
 |
1 & 8 & 20 mg/ml |
25°C |
 |
| 30 |
 |
|
800 |
 Fosphenytoin sodium Fosphenytoin sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 30 |
 |
|
800 |
 Furosemide Furosemide
|
 |
 |
|
2-8°C |
 |
| 14 |
 |
|
3810 |
 Fusidate sodium Fusidate sodium
|
 |
 |
0.5 & 1 mg/ml |
25°C |
 |
| 48 |
 |
|
3661 |
 Gabapentine Gabapentine
|
 |
 |
mg/ml |
18-26°C |
 |
| 98 |
 |
|
3322 |
 Gabapentine Gabapentine
|
 |
 |
mg/ml |
2-8°C |
 |
| 125 |
 |
|
3322 |
 Gabapentine Gabapentine
|
 |
 |
mg/ml |
25°C |
 |
| 56 |
 |
|
2759 |
 Gabapentine Gabapentine
|
 |
 |
mg/ml |
25°C |
 |
| 56 |
 |
|
2759 |
 Gabapentine Gabapentine
|
 |
 |
mg/ml |
4°C |
 |
| 91 |
 |
|
2759 |
 Gabapentine Gabapentine
|
 |
 |
mg/ml |
4°C |
 |
| 56 |
 |
|
2759 |
 Gallium nitrate Gallium nitrate
|
 |
 |
0,22 & 0,6 mg/ml |
20°C |
 |
| 14 |
 |
|
141 |
 Gallium nitrate Gallium nitrate
|
 |
 |
0,22 & 0,6 mg/ml |
4°C |
 |
| 14 |
 |
|
141 |
 Ganciclovir sodium Ganciclovir sodium
|
 |
 |
20 mg/ml |
-8°C |
 |
| 24 |
 |
|
3796 |
 Ganciclovir sodium Ganciclovir sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
3796 |
 Ganciclovir sodium Ganciclovir sodium
|
 |
 |
20 mg/ml |
5°C |
 |
| 24 |
 |
|
3796 |
 Ganciclovir sodium Ganciclovir sodium
|
 |
 |
1 & 5 mg/ml |
25°C |
 |
| 35 |
 |
|
76 |
 Ganciclovir sodium Ganciclovir sodium
|
 |
 |
0,28 & 1,4 mg/ml |
25°C |
 |
| 8 |
 |
|
245 |
 Ganciclovir sodium Ganciclovir sodium
|
 |
 |
1,4 & 7 mg/ml |
25°C |
 |
| 8 |
 |
|
245 |
 Ganciclovir sodium Ganciclovir sodium
|
 |
 |
1 & 6 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
5,12 mg/ml |
22°C |
 |
| 48 |
 |
|
1520 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
5,5>>6,6 mg/ml |
2-8°C |
 |
| 49 |
 |
|
4736 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
5,5>>6,6 mg/ml |
21-25°C |
 |
| 49 |
 |
|
4736 |
 Gentamicin sulfate Gentamicin sulfate
|
 |
 |
mg:ml |
26°C |
 |
| 7 |
 |
|
2547 |
 Gentamicin sulfate Gentamicin sulfate
|
 |
 |
mg:ml |
26°C |
 |
| 7 |
 |
|
2547 |
 Gentamicin sulfate Gentamicin sulfate
|
 |
 |
0,5 & 5 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Gentamicin sulfate Gentamicin sulfate
|
 |
 |
0,5 & 5 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Gentamicin sulfate Gentamicin sulfate
|
 |
 |
2,5 mg/ml |
37°C |
 |
| 96 |
 |
|
3952 |
 Glycopyrronium bromide Glycopyrronium bromide
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
4198 |
 Glycopyrronium bromide Glycopyrronium bromide
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
4198 |
 Granisetron hydrochloride Granisetron hydrochloride
|
 |
 |
0,02 & 0,06 mg/ml |
23°C |
 |
| 35 |
 |
|
1633 |
 Granisetron hydrochloride Granisetron hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 14 |
 |
|
2427 |
 Granisetron hydrochloride Granisetron hydrochloride
|
 |
 |
mg/ml |
5°C |
 |
| 14 |
 |
|
2427 |
 Heparin sodium Heparin sodium
|
 |
 |
1 UI/ml |
20°C-25°C |
 |
| 365 |
 |
|
1291 |
 Heparin sodium Heparin sodium
|
 |
 |
10 UI/ml |
20°C-25°C |
 |
| 365 |
 |
|
1291 |
 Heparin sodium Heparin sodium
|
 |
 |
1 UI/ml |
20°C |
 |
| 365 |
 |
|
1234 |
 Heparin sodium Heparin sodium
|
 |
 |
1 UI/ml |
37°C |
 |
| 365 |
 |
|
1234 |
 Heparin sodium Heparin sodium
|
 |
 |
1000 & 40000 UI/ml |
30°C |
 |
| 30 |
 |
|
874 |
 Hydralazine hydrochloride Hydralazine hydrochloride
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 1 |
 |
|
2367 |
 Hydrochlorothiazide Hydrochlorothiazide
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Hydrocortisone Hydrocortisone
|
 |
 |
mg |
25°C |
 |
| 90 |
 |
|
4198 |
 Hydrocortisone Hydrocortisone
|
 |
 |
mg |
4°C |
 |
| 90 |
 |
|
4198 |
 Hydrocortisone Hydrocortisone
|
 |
 |
mg |
2-8°C |
 |
| 60 |
 |
|
3810 |
 Hydrocortisone Hydrocortisone
|
 |
 |
mg |
25°C |
 |
| 60 |
 |
|
3810 |
 Hydrocortisone sodium phosphate Hydrocortisone sodium phosphate
|
 |
 |
mg/ml |
2-8°C |
 |
| 60 |
 |
|
3810 |
 Hydrocortisone sodium phosphate Hydrocortisone sodium phosphate
|
 |
 |
mg/ml |
25°C |
 |
| 60 |
 |
|
3810 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
2 mg/ml |
23°C |
 |
| 24 |
 |
|
31 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
1 mg/ml |
7°C |
 |
| 15 |
 |
|
2001 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
1 mg/ml |
7°C |
 |
| 15 |
 |
|
2001 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
50 mg/ml |
5°C |
 |
| 120 |
 |
|
2001 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
10 mg/ml |
25°C |
 |
| 3 |
 |
|
2354 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
10 mg/ml |
5°C |
 |
| 21 |
 |
|
2354 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
2 mg/ml |
23°C |
 |
| 24 |
 |
|
31 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
1 & 5 mg/ml |
23°C |
 |
| 42 |
 |
|
120 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
0,2 & 10 mg/ml |
23°C |
 |
| 90 |
 |
|
4230 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
20 mg/ml |
22°C |
 |
| 7 |
 |
|
4664 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
20 mg/ml |
37°C |
 |
| 7 |
 |
|
4664 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
0,2 & 10 mg/ml |
23°C |
 |
| 90 |
 |
|
4230 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 100 |
 |
|
4303 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
0,1 mg/ml |
30°C |
 |
| 30 |
 |
|
5 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
10 mg/ml |
30°C |
 |
| 30 |
 |
|
5 |
 Hydroxocobalamin Hydroxocobalamin
|
 |
 |
25 mg/ml |
2-8°C |
 |
| 6 |
 |
|
3932 |
 Hydroxocobalamin Hydroxocobalamin
|
 |
 |
25 mg/ml |
25°C |
 |
| 6 |
 |
|
3932 |
 Ifosfamide Ifosfamide
|
 |
 |
80 mg/ml |
35°C |
 |
| 8 |
 |
|
489 |
 Ifosfamide Ifosfamide
|
 |
 |
20 & 40 & 80 mg/ml |
35°C |
 |
| 8 |
 |
|
489 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
2.5 & 5 mg/ml |
25°C |
 |
| 9 |
 |
|
21 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
2.5 & 5 mg/ml |
25°C |
 |
| 9 |
 |
|
21 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
2.5 mg/ml |
25°C |
 |
| 6 |
 |
|
21 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
5 mg/ml |
25°C |
 |
| 3 |
 |
|
21 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
2.5 mg/ml |
25°C |
 |
| 6 |
 |
|
21 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
5 mg/ml |
25°C |
 |
| 3 |
 |
|
21 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
2.5 & 5 mg/ml |
25°C |
 |
| 3 |
 |
|
21 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
5 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
8 mg/ml |
25°C |
 |
| 3 |
 |
|
2042 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
8 mg/ml |
37°C |
 |
| 2 |
 |
|
2042 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 8 |
 |
|
4298 |
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
5 mg/ml |
23-27°C |
 |
| 4 |
 |
|
4298 |
 Indocyanine green Indocyanine green
|
 |
 |
2,5 mg/ml |
-20°C |
 |
| 28 |
 |
|
4246 |
 Indocyanine green Indocyanine green
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 30 |
 |
|
4246 |
 Indocyanine green Indocyanine green
|
 |
 |
2,5 mg/ml |
4°C |
 |
| 32 |
 |
|
4246 |
 Indomethacin sodium trihydrate Indomethacin sodium trihydrate
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 10 |
 |
|
923 |
 Infliximab Infliximab
|
 |
 |
10 mg/ml |
2-8°C |
 |
| 28 |
 |
|
754 |
 Infliximab Infliximab
|
 |
 |
10 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4279 |
 Infliximab Infliximab
|
 |
 |
10 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4279 |
 Infliximab Infliximab
|
 |
 |
10 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4292 |
 Infliximab Infliximab
|
 |
 |
10 mg/ml |
23-27°C |
 |
| 14 |
 |
|
4292 |
 Infliximab Infliximab
|
 |
 |
0.4 mg/ml |
4°C |
 |
| 14 |
 |
|
3333 |
 Infliximab Infliximab
|
 |
 |
0,4 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4292 |
 Infliximab Infliximab
|
 |
 |
0,4 mg/ml |
23-27°C |
 |
| 30 |
 |
|
4292 |
 Infliximab Infliximab
|
 |
 |
4 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4292 |
 Infliximab Infliximab
|
 |
 |
4 mg/ml |
23-27°C |
 |
| 30 |
 |
|
4292 |
 Infliximab Infliximab
|
 |
 |
0,4 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4279 |
 Infliximab Infliximab
|
 |
 |
0,4 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4279 |
 Infliximab Infliximab
|
 |
 |
0,7 mg/ml |
22°C |
 |
| 30 |
 |
|
3317 |
 Infliximab Infliximab
|
 |
 |
0,7 mg/ml |
4°C |
 |
| 30 |
 |
|
3317 |
 Infliximab Infliximab
|
 |
 |
1,6 mg/ml |
22°C |
 |
| 30 |
 |
|
3317 |
 Infliximab Infliximab
|
 |
 |
1,6 mg/ml |
4°C |
 |
| 30 |
 |
|
3317 |
 Infliximab Infliximab
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4279 |
 Infliximab Infliximab
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4279 |
 Infliximab Infliximab
|
 |
 |
< 4 mg/ml |
2-8°C |
 |
| 28 |
 |
|
754 |
 Infliximab Infliximab
|
 |
 |
mg |
-20°C |
 |
| 45 |
 |
|
3754 |
 Infliximab Infliximab
|
 |
 |
mg |
4°C |
 |
| 45 |
 |
|
3754 |
 Insulin Insulin
|
 |
 |
0,004 UI/ml |
25°C |
 |
| 24 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,01 UI/ml |
25°C |
 |
| 24 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,02 UI/ml |
25°C |
 |
| 24 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,04 UI/ml |
25°C |
 |
| 24 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,01 UI/ml |
25°C |
 |
| 6 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,02 UI/ml |
25°C |
 |
| 6 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,04 UI/ml |
25°C |
 |
| 6 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,01 UI/ml |
25°C |
 |
| 6 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,02 UI/ml |
25°C |
 |
| 6 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
0,04 UI/ml |
25°C |
 |
| 6 |
 |
|
2164 |
 Insulin Insulin
|
 |
 |
1 UI/ml |
23-27°C |
 |
| 30 |
 |
|
3224 |
 Insulin Insulin
|
 |
 |
500 UI/ml |
4°C |
 |
| 28 |
 |
|
4309 |
 Insulin glulisine Insulin glulisine
|
 |
 |
1 U/ml |
25°C |
 |
| 48 |
 |
|
3935 |
 Ipilimumab Ipilimumab
|
 |
 |
1 >> 4 mg/ml |
25°C |
 |
| 24 |
 |
|
3352 |
 Ipilimumab Ipilimumab
|
 |
 |
1 >> 4 mg/ml |
25°C |
 |
| 24 |
 |
|
3352 |
 Ipilimumab Ipilimumab
|
 |
 |
1 >> 4 mg/ml |
25°C |
 |
| 24 |
 |
|
3352 |
 Irinotecan Irinotecan
|
 |
 |
0,02 mg/ml |
25°C |
 |
| 1 |
 |
|
1617 |
 Irinotecan Irinotecan
|
 |
 |
0,02 mg/ml |
25°C |
 |
| 24 |
 |
|
1617 |
 Irinotecan Irinotecan
|
 |
 |
mg/ml |
5°C |
 |
| 28 |
 |
|
3129 |
 Irinotecan Irinotecan
|
 |
 |
mg/ml |
5°C |
 |
| 28 |
 |
|
3129 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
15-25°C |
 |
| 8 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 48 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
15-25°C |
 |
| 8 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 48 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
15-25°C |
 |
| 8 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 48 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
15-25°C |
 |
| 8 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 48 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
15-25°C |
 |
| 8 |
 |
|
4617 |
 Isatuximab Isatuximab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 48 |
 |
|
4617 |
 Isavuconazonium sulfate Isavuconazonium sulfate
|
 |
 |
10 mg/ml |
25°C |
 |
| 1 |
 |
|
2864 |
 Isavuconazonium sulfate Isavuconazonium sulfate
|
 |
 |
20 mg/ml |
25°C |
 |
| 1 |
 |
|
2864 |
 Isavuconazonium sulfate Isavuconazonium sulfate
|
 |
 |
1,5 mg/ml |
2-8°C |
 |
| 24 |
 |
|
2864 |
 Isavuconazonium sulfate Isavuconazonium sulfate
|
 |
 |
1,5 mg/ml |
25°C |
 |
| 6 |
 |
|
2864 |
 Isoprenaline hydrochloride Isoprenaline hydrochloride
|
 |
 |
? mg/ml |
25°C |
 |
| 24 |
 |
|
3660 |
 Itraconazole Itraconazole
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
4198 |
 Itraconazole Itraconazole
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
4198 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 28 |
 |
|
3859 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
1 mg/ml |
33°C |
 |
| 7 |
 |
|
3859 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 30 |
 |
|
1714 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 365 |
 |
|
2287 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
1 mg/ml |
40°C |
 |
| 365 |
 |
|
2287 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
1 mg/ml |
4°C |
 |
| 365 |
 |
|
2287 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 100 |
 |
|
4303 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
50 mg/ml |
25°C |
 |
| 50 |
 |
|
3911 |
 Ketorolac tromethamine Ketorolac tromethamine
|
 |
 |
0,3 & 0,6 mg/ml |
23°C |
 |
| 21 |
 |
|
1634 |
 Ketorolac tromethamine Ketorolac tromethamine
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 35 |
 |
|
451 |
 Ketorolac tromethamine Ketorolac tromethamine
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 7 |
 |
|
451 |
 L-Methionine L-Methionine
|
 |
 |
mg/mL |
2-6°c |
 |
| 45 |
 |
|
4747 |
 L-Methionine L-Methionine
|
 |
 |
mg/mL |
21-25°c |
 |
| 45 |
 |
|
4747 |
 Labetalol hydrochloride Labetalol hydrochloride
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 72 |
 |
|
1194 |
 Labetalol hydrochloride Labetalol hydrochloride
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 72 |
 |
|
1194 |
 Labetalol hydrochloride Labetalol hydrochloride
|
 |
 |
mg |
25°C |
 |
| 90 |
 |
|
4198 |
 Labetalol hydrochloride Labetalol hydrochloride
|
 |
 |
mg |
4°C |
 |
| 90 |
 |
|
4198 |
 Lenograstim Lenograstim
|
 |
 |
2,63 µg/ml |
4°C |
 |
| 14 |
 |
|
3760 |
 Levetiracetam Levetiracetam
|
 |
 |
40 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3847 |
 Levetiracetam Levetiracetam
|
 |
 |
40 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3847 |
 Levetiracetam Levetiracetam
|
 |
 |
40 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3847 |
 Levodopa/Carbidopa Levodopa/Carbidopa
|
 |
 |
mg/ml |
25°C |
 |
| 28 |
 |
|
2721 |
 Levodopa/Carbidopa Levodopa/Carbidopa
|
 |
 |
mg/ml |
4°C |
 |
| 42 |
 |
|
2721 |
 Levofolinate calcium Levofolinate calcium
|
 |
 |
0,2 & 0,7 mg/ml |
4°C |
 |
| 8 |
 |
|
1520 |
 Levosimendan Levosimendan
|
 |
 |
0,025 & 0,05 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4571 |
 Levosimendan Levosimendan
|
 |
 |
0,025 & 0,05 mg/ml |
25°C |
 |
| 24 |
 |
|
4571 |
 Levothyroxine Levothyroxine
|
 |
 |
mg/ml |
22°C |
 |
| 28 |
 |
|
4112 |
 Levothyroxine Levothyroxine
|
 |
 |
mg/ml |
22°C |
 |
| 28 |
 |
|
4112 |
 Levothyroxine Levothyroxine
|
 |
 |
mg/ml |
25°C |
 |
| 15 |
 |
|
2657 |
 Levothyroxine Levothyroxine
|
 |
 |
mg/ml |
4°C |
 |
| 28 |
 |
|
4112 |
 Levothyroxine Levothyroxine
|
 |
 |
mg/ml |
4°C |
 |
| 28 |
 |
|
4112 |
 Levothyroxine Levothyroxine
|
 |
 |
mg/ml |
5°C |
 |
| 47 |
 |
|
2657 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
0.45 mg/ml |
22°C |
 |
| 21 |
 |
|
1126 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
0.45 mg/ml |
22°C |
 |
| 21 |
 |
|
1126 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
4 mg/ml |
30°C |
 |
| 120 |
 |
|
1202 |
 Linezolid Linezolid
|
 |
 |
0,26 mg/ml |
37°C |
 |
| 24 |
 |
|
4176 |
 Linezolid Linezolid
|
 |
 |
0,21 mg/ml |
37°C |
 |
| 24 |
 |
|
4176 |
 Linezolid Linezolid
|
 |
 |
0,26 mg/ml |
37°C |
 |
| 24 |
 |
|
4176 |
 Linezolid Linezolid
|
 |
 |
0,26 mg/ml |
37°C |
 |
| 24 |
 |
|
4176 |
 Linezolid Linezolid
|
 |
 |
2 mg/ml |
25°C |
 |
| 34 |
 |
|
4117 |
 Linezolid Linezolid
|
 |
 |
2 mg/ml |
25°C |
 |
| 34 |
 |
|
4117 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
23°C |
 |
| 30 |
 |
|
2680 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
23°C |
 |
| 30 |
 |
|
2665 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
4°C |
 |
| 30 |
 |
|
2665 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
5°C |
 |
| 30 |
 |
|
2680 |
 Lorazepam Lorazepam
|
 |
 |
0,167 mg/ml |
2-8°C |
 |
| 1 |
 |
|
4248 |
 Lorazepam Lorazepam
|
 |
 |
0,167 mg/ml |
20-25°C |
 |
| 1 |
 |
|
4248 |
 Lorazepam Lorazepam
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
935 |
 Lorazepam Lorazepam
|
 |
 |
0,91 mg/ml |
24°C |
 |
| 35 |
 |
|
1935 |
 Lorazepam Lorazepam
|
 |
 |
0,91 mg/ml |
37°C |
 |
| 5 |
 |
|
1935 |
 Lorazepam Lorazepam
|
 |
 |
0,91 mg/ml |
4°C |
 |
| 35 |
 |
|
1935 |
 Lorazepam Lorazepam
|
 |
 |
0,167 mg/ml |
2-8°C |
 |
| 2 |
 |
|
4248 |
 Lorazepam Lorazepam
|
 |
 |
0,167 mg/ml |
20-25°C |
 |
| 4 |
 |
|
4248 |
 Magnesium sulfate Magnesium sulfate
|
 |
 |
40 mg/ml |
19-24°C |
 |
| 90 |
 |
|
2356 |
 Magnesium sulfate Magnesium sulfate
|
 |
 |
40 mg/ml |
19-24°C |
 |
| 90 |
 |
|
2356 |
 Magnesium sulfate Magnesium sulfate
|
 |
 |
40 mg/ml |
19-24°C |
 |
| 90 |
 |
|
2356 |
 Magnesium sulfate Magnesium sulfate
|
 |
 |
40 mg/ml |
19-24°C |
 |
| 90 |
 |
|
2356 |
 Magnesium sulfate Magnesium sulfate
|
 |
 |
200 mg/ml |
22°C |
 |
| 30 |
 |
|
2294 |
 Magnesium sulfate Magnesium sulfate
|
 |
 |
200 mg/ml |
4-6°C |
 |
| 60 |
 |
|
2294 |
 Melatonine Melatonine
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
4198 |
 Melatonine Melatonine
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
4198 |
 Melatonine Melatonine
|
 |
 |
mg |
23-27°C |
 |
| 547 |
 |
|
4079 |
 Melatonine Melatonine
|
 |
 |
mg |
23-27°C |
 |
| 547 |
 |
|
4079 |
 Melatonine Melatonine
|
 |
 |
mg |
23-27°C |
 |
| 547 |
 |
|
4079 |
 Melphalan Melphalan
|
 |
 |
5 mg/ml |
23°C |
 |
| 6 |
 |
|
4189 |
 Melphalan Melphalan
|
 |
 |
1 mg/ml |
23°C |
 |
| 1 |
 |
|
4189 |
 Melphalan Melphalan
|
 |
 |
1 mg/ml |
4°C |
 |
| 2 |
 |
|
4189 |
 Melphalan Melphalan
|
 |
 |
1,5 & 2 mg/ml |
23°C |
 |
| 2 |
 |
|
4189 |
 Melphalan Melphalan
|
 |
 |
1,5 & 2 mg/ml |
4°C |
 |
| 2 |
 |
|
4189 |
 Melphalan Melphalan
|
 |
 |
0,06 mg/ml |
21°C-22°C |
 |
| 2 |
 |
|
1520 |
 Melphalan Melphalan
|
 |
 |
0,06 mg/ml |
4°C |
 |
| 24 |
 |
|
1520 |
 Melphalan Melphalan
|
 |
 |
0,015 mg/ml |
-18°C |
 |
| 28 |
 |
|
3949 |
 Melphalan Melphalan
|
 |
 |
0,2 mg/ml |
-18°C |
 |
| 28 |
 |
|
3949 |
 Melphalan captisol Melphalan captisol
|
 |
 |
5 mg/ml |
23°C |
 |
| 24 |
 |
|
4189 |
 Melphalan captisol Melphalan captisol
|
 |
 |
5 mg/ml |
4°C |
 |
| 24 |
 |
|
4189 |
 Melphalan captisol Melphalan captisol
|
 |
 |
1 mg/ml |
23°C |
 |
| 6 |
 |
|
4189 |
 Melphalan captisol Melphalan captisol
|
 |
 |
1 mg/ml |
4°C |
 |
| 8 |
 |
|
4189 |
 Melphalan captisol Melphalan captisol
|
 |
 |
2 mg/ml |
23°C |
 |
| 24 |
 |
|
4189 |
 Melphalan captisol Melphalan captisol
|
 |
 |
2 mg/ml |
4°C |
 |
| 24 |
 |
|
4189 |
 Menadione Menadione
|
 |
 |
mg |
25°C |
 |
| 60 |
 |
|
2988 |
 Mercaptopurine Mercaptopurine
|
 |
 |
mg |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Mercaptopurine Mercaptopurine
|
 |
 |
mg |
20-25°C |
 |
| 90 |
 |
|
4177 |
 Meropenem Meropenem
|
 |
 |
2,5 mg/ml |
21°C-26°C |
 |
| 24 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
40 mg/ml |
25°C |
 |
| 12 |
 |
|
3112 |
 Meropenem Meropenem
|
 |
 |
50 mg/ml |
21-26°C |
 |
| 8 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
50 mg/ml |
23-27°C |
 |
| 7 |
 |
|
4403 |
 Meropenem Meropenem
|
 |
 |
2,5 mg/ml |
21-26°C |
 |
| 24 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
50 mg/ml |
21-26°C |
 |
| 8 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
2,5 & 50 mg/ml |
21-26°C |
 |
| 3 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
1 mg/ml |
21-26°C |
 |
| 24 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
20 mg/ml |
21-26°C |
 |
| 10 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
1 mg/ml |
21°C-26°C |
 |
| 24 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
10 mg/ml |
25°C |
 |
| 12 |
 |
|
4290 |
 Meropenem Meropenem
|
 |
 |
10 & 20 mg/ml |
5°C |
 |
| 5 |
 |
|
1942 |
 Meropenem Meropenem
|
 |
 |
14,2 mg/ml |
22°C |
 |
| 7 |
 |
|
4122 |
 Meropenem Meropenem
|
 |
 |
14,2 mg/ml |
33°C |
 |
| 5 |
 |
|
4122 |
 Meropenem Meropenem
|
 |
 |
20 mg/ml |
21-26°C |
 |
| 10 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
4 mg/ml |
5°C |
 |
| 7 |
 |
|
1942 |
 Meropenem Meropenem
|
 |
 |
1 mg/ml |
21-26°C |
 |
| 4 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
20 mg/ml |
21-26°C |
 |
| 3 |
 |
|
10 |
 Meropenem Meropenem
|
 |
 |
10 mg/ml |
25°C |
 |
| 12 |
 |
|
4147 |
 Meropenem Meropenem
|
 |
 |
20 mg/ml |
22°C |
 |
| 17 |
 |
|
4592 |
 Meropenem Meropenem
|
 |
 |
20 mg/ml |
25°C |
 |
| 12 |
 |
|
4147 |
 Meropenem Meropenem
|
 |
 |
40 mg/ml |
25°C |
 |
| 8 |
 |
|
4147 |
 Meropenem Meropenem
|
 |
 |
10 & 20 mg/ml |
5°C |
 |
| 5 |
 |
|
1942 |
 Meropenem Meropenem
|
 |
 |
4 mg/ml |
5°C |
 |
| 7 |
 |
|
1942 |
 Meropenem Meropenem
|
 |
 |
64 mg/ml |
25°C |
 |
| 5 |
 |
|
2042 |
 Meropenem Meropenem
|
 |
 |
64 mg/ml |
37°C |
 |
| 1 |
 |
|
2042 |
 Meropenem Meropenem
|
 |
 |
5 mg/ml |
20°C |
 |
| 8 |
 |
|
3837 |
 Meropenem Meropenem
|
 |
 |
5 mg/ml |
23-27°C |
 |
| 12 |
 |
|
4403 |
 Meropenem Meropenem
|
 |
 |
5 mg/ml |
32-37°C |
 |
| 4 |
 |
|
3837 |
 Meropenem Meropenem
|
 |
 |
5 mg/ml |
4°C |
 |
| 24 |
 |
|
4403 |
 Meropenem Meropenem
|
 |
 |
5 mg/ml |
23-27°C |
 |
| 4 |
 |
|
4403 |
 Methadone hydrochloride Methadone hydrochloride
|
 |
 |
5 mg/ml |
23-27°C |
 |
| 180 |
 |
|
4406 |
 Methadone hydrochloride Methadone hydrochloride
|
 |
 |
5 mg/ml |
5°C |
 |
| 180 |
 |
|
4406 |
 Methadone hydrochloride Methadone hydrochloride
|
 |
 |
mg |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Methotrexate sodium Methotrexate sodium
|
 |
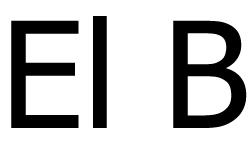 |
2 mg/ml |
23°C |
 |
| 48 |
 |
|
31 |
 Methotrexate sodium Methotrexate sodium
|
 |
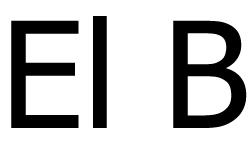 |
2 mg/ml |
4°C |
 |
| 48 |
 |
|
31 |
 Methotrexate sodium Methotrexate sodium
|
 |
 |
0,36 mg/ml |
21°C-22°C |
 |
| 48 |
 |
|
1520 |
 Methotrexate sodium Methotrexate sodium
|
 |
 |
0,36 mg/ml |
4°C |
 |
| 48 |
 |
|
1520 |
 Methotrexate sodium Methotrexate sodium
|
 |
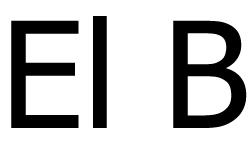 |
2 mg/ml |
23°C |
 |
| 48 |
 |
|
31 |
 Methylprednisolone sodium succinate Methylprednisolone sodium succinate
|
 |
 |
10 mg/ml |
25°C |
 |
| 4 |
 |
|
1627 |
 Methylprednisolone sodium succinate Methylprednisolone sodium succinate
|
 |
 |
10 mg/ml |
5°C |
 |
| 21 |
 |
|
1627 |
 Metoclopramide hydrochloride Metoclopramide hydrochloride
|
 |
 |
0,5 mg/ml |
25°C |
 |
| 21 |
 |
|
1957 |
 Metoclopramide hydrochloride Metoclopramide hydrochloride
|
 |
 |
5 mg/ml |
23°C |
 |
| 60 |
 |
|
180 |
 Metoclopramide hydrochloride Metoclopramide hydrochloride
|
 |
 |
5 mg/ml |
32°C |
 |
| 7 |
 |
|
180 |
 Metolazone Metolazone
|
 |
 |
mg/ml |
25°C |
 |
| 7 |
 |
|
2634 |
 Metolazone Metolazone
|
 |
 |
mg/ml |
25°C |
 |
| 7 |
 |
|
2634 |
 Metoprolol tartrate Metoprolol tartrate
|
 |
 |
0.5 mg/ml |
23-25°C |
 |
| 30 |
 |
|
2267 |
 Metoprolol tartrate Metoprolol tartrate
|
 |
 |
1 mg/ml |
23-25°C |
 |
| 30 |
 |
|
2267 |
 Metronidazole Metronidazole
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
2663 |
 Mexiletine Mexiletine
|
 |
 |
mg/ml |
25°C |
 |
| 49 |
 |
|
2690 |
 Mexiletine Mexiletine
|
 |
 |
mg/ml |
25°C |
 |
| 14 |
 |
|
2690 |
 Mexiletine Mexiletine
|
 |
 |
mg/ml |
4°C |
 |
| 91 |
 |
|
2690 |
 Mexiletine Mexiletine
|
 |
 |
mg/ml |
4°C |
 |
| 28 |
 |
|
2690 |
 Micafungin Micafungin
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
3573 |
 Micafungin Micafungin
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
3573 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
1 mg/ml |
-20°C |
 |
| 365 |
 |
|
4201 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 365 |
 |
|
4201 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
1 mg/ml |
23-25°C |
 |
| 90 |
 |
|
4201 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
2 mg/ml |
30°C |
 |
| 10 |
 |
|
5 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
2 mg/ml |
5°C |
 |
| 10 |
 |
|
5 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
3 mg/ml |
32°C |
 |
| 7 |
 |
|
401 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
0,4 mg/ml |
25°C |
 |
| 100 |
 |
|
4303 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
5 mg/ml |
25°C |
 |
| 100 |
 |
|
4303 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
1 mg/ml |
-20°C |
 |
| 365 |
 |
|
4201 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 365 |
 |
|
4201 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
1 mg/ml |
23-25°C |
 |
| 90 |
 |
|
4201 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
|
23°C |
 |
| 102 |
 |
|
2575 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,2 mg/ml |
20-25°C |
 |
| 14 |
 |
|
922 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,2 & 0,025 mg/ml |
25°C |
 |
| 90 |
 |
|
4216 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,2 & 0,025 mg/ml |
4°C |
 |
| 90 |
 |
|
4216 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,2 & 0,025 mg/ml |
25°C |
 |
| 90 |
 |
|
4216 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,2 & 0,025 mg/ml |
4°C |
 |
| 90 |
 |
|
4216 |
 Milrinone lactate Milrinone lactate
|
 |
 |
1 mg/ml |
25°C |
 |
| 90 |
 |
|
4216 |
 Milrinone lactate Milrinone lactate
|
 |
 |
1 mg/ml |
4°C |
 |
| 90 |
 |
|
4216 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 24 |
 |
|
4109 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,6 mg/ml |
35°C |
 |
| 24 |
 |
|
4109 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,6 mg/ml |
4°C |
 |
| 7 |
 |
|
4109 |
 Minocycline hydrochloride Minocycline hydrochloride
|
 |
 |
0,1 mg/ml |
24°C |
 |
| 7 |
 |
|
263 |
 Minocycline hydrochloride Minocycline hydrochloride
|
 |
 |
0,1 mg/ml |
4°C |
 |
| 7 |
 |
|
263 |
 Mitomycin Mitomycin
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
1827 |
 Mitomycin Mitomycin
|
 |
 |
1 mg/ml |
37°C |
 |
| 5 |
 |
|
4282 |
 Mitomycin Mitomycin
|
 |
 |
2 mg/ml |
37°C |
 |
| 5 |
 |
|
4282 |
 Mitomycin Mitomycin
|
 |
 |
0,4 mg/ml |
-20°C |
 |
| 23 |
 |
|
4367 |
 Mitomycin Mitomycin
|
 |
 |
0,4 mg/ml |
25°C |
 |
| 24 |
 |
|
4367 |
 Mitomycin Mitomycin
|
 |
 |
0,4 mg/ml |
4°C |
 |
| 14 |
 |
|
4367 |
 Mitoxantrone dihydrochloride Mitoxantrone dihydrochloride
|
 |
 |
0,04 & 0,4 mg/ml |
23°C |
 |
| 7 |
 |
|
828 |
 Mitoxantrone dihydrochloride Mitoxantrone dihydrochloride
|
 |
 |
0,2 mg/ml |
20°C |
 |
| 28 |
 |
|
1317 |
 Morphine hydrochloride Morphine hydrochloride
|
 |
 |
0,5 >> 2,5 mg/ml |
32°C |
 |
| 60 |
 |
|
679 |
 Morphine hydrochloride Morphine hydrochloride
|
 |
 |
25 mg/ml |
22°C |
 |
| 7 |
 |
|
4664 |
 Morphine hydrochloride Morphine hydrochloride
|
 |
 |
25 mg/ml |
37°C |
 |
| 7 |
 |
|
4664 |
 Morphine hydrochloride Morphine hydrochloride
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 58 |
 |
|
3572 |
 Morphine hydrochloride Morphine hydrochloride
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 58 |
 |
|
3572 |
 Morphine sulfate Morphine sulfate
|
 |
 |
2 & 15 mg/ml |
23°C-25°C |
 |
| 12 |
 |
|
65 |
 Morphine sulfate Morphine sulfate
|
 |
 |
2 & 15 mg/ml |
23°C-25°C |
 |
| 12 |
 |
|
65 |
 Morphine sulfate Morphine sulfate
|
 |
 |
5 mg/ml |
23°C |
 |
| 30 |
 |
|
120 |
 Morphine sulfate Morphine sulfate
|
 |
 |
10 mg/ml |
23°C |
 |
| 31 |
 |
|
121 |
 Morphine sulfate Morphine sulfate
|
 |
 |
10 mg/ml |
4°C |
 |
| 31 |
 |
|
121 |
 Morphine sulfate Morphine sulfate
|
 |
 |
10 mg/ml |
23°C |
 |
| 31 |
 |
|
121 |
 Morphine sulfate Morphine sulfate
|
 |
 |
25 & 50 mg/ml |
23°C |
 |
| 31 |
 |
|
121 |
 Morphine sulfate Morphine sulfate
|
 |
 |
1 mg/ml |
25°C |
 |
| 1095 |
 |
|
2064 |
 Morphine sulfate Morphine sulfate
|
 |
 |
1 mg/ml |
25°C |
 |
| 100 |
 |
|
4303 |
 Morphine sulfate Morphine sulfate
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 91 |
 |
|
4395 |
 Morphine sulfate Morphine sulfate
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 91 |
 |
|
4395 |
 Morphine sulfate Morphine sulfate
|
 |
 |
1,0 mg/ml |
25°C |
 |
| 91 |
 |
|
4395 |
 Morphine sulfate Morphine sulfate
|
 |
 |
0,02 mg/ml |
25°C |
 |
| 91 |
 |
|
4395 |
 Morphine sulfate Morphine sulfate
|
 |
 |
0,04 mg/ml |
25°C |
 |
| 91 |
 |
|
4395 |
 Morphine sulfate Morphine sulfate
|
 |
 |
2 & 15 mg/ml |
23°C-25°C |
 |
| 12 |
 |
|
65 |
 Morphine sulfate Morphine sulfate
|
 |
 |
2 & 15 mg/ml |
31°C |
 |
| 12 |
 |
|
65 |
 Morphine sulfate Morphine sulfate
|
 |
 |
2 & 10 mg/ml |
22°C |
 |
| 40 |
 |
|
1810 |
 Morphine sulfate Morphine sulfate
|
 |
 |
2 & 10 mg/ml |
4°C |
 |
| 40 |
 |
|
1810 |
 Morphine sulfate Morphine sulfate
|
 |
 |
2 & 3 mg/ml |
25°C |
 |
| 60 |
 |
|
2123 |
 Morphine sulfate Morphine sulfate
|
 |
 |
2 & 3 mg/ml |
4°C |
 |
| 60 |
 |
|
2123 |
 Moxalactam Moxalactam
|
 |
 |
20 & 333 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxalactam Moxalactam
|
 |
 |
2 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxalactam Moxalactam
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxalactam Moxalactam
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxalactam Moxalactam
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxalactam Moxalactam
|
 |
 |
333 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxalactam Moxalactam
|
 |
 |
333 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxalactam Moxalactam
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxalactam Moxalactam
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
626 |
 Moxifloxacin Moxifloxacin
|
 |
 |
0.025 mg/ml |
25°C |
 |
| 7 |
 |
|
2145 |
 Moxifloxacin Moxifloxacin
|
 |
 |
0.025 mg/ml |
37°C |
 |
| 3 |
 |
|
2145 |
 Moxifloxacin Moxifloxacin
|
 |
 |
0.025 mg/ml |
4°C |
 |
| 14 |
 |
|
2145 |
 Moxifloxacin Moxifloxacin
|
 |
 |
0.025 mg/ml |
25°C |
 |
| 3 |
 |
|
2145 |
 Moxifloxacin Moxifloxacin
|
 |
 |
0.025 mg/ml |
37°C |
 |
| 12 |
 |
|
2145 |
 Moxifloxacin Moxifloxacin
|
 |
 |
0.025 mg/ml |
4°C |
 |
| 14 |
 |
|
2145 |
 Mycophenolate mofetil Mycophenolate mofetil
|
 |
 |
1 & 10 mg/ml |
25°C |
 |
| 7 |
 |
|
1981 |
 Mycophenolate mofetil Mycophenolate mofetil
|
 |
 |
1 & 10 mg/ml |
4°C |
 |
| 7 |
 |
|
1981 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
26 mg/ml |
25°C |
 |
| 60 |
 |
|
2076 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
2558 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
mg/ml |
2-8°C |
 |
| 35 |
 |
|
2426 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
mg/ml |
23-25°C |
 |
| 30 |
 |
|
2426 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
mg/ml |
25°C |
 |
| 41 |
 |
|
2558 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
25 mg/ml |
23-27°C |
 |
| 24 |
 |
|
4727 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
mg |
25°C |
 |
| 60 |
 |
|
3165 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
mg |
25°C |
 |
| 60 |
 |
|
3165 |
 Nafcillin sodium Nafcillin sodium
|
 |
 |
20 & 120 mg/ml |
25°C |
 |
| 3 |
 |
|
13 |
 Nafcillin sodium Nafcillin sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 7 |
 |
|
2352 |
 Nafcillin sodium Nafcillin sodium
|
 |
 |
10 mg/ml |
5°C |
 |
| 44 |
 |
|
2352 |
 Nalidixic acid Nalidixic acid
|
 |
 |
mg |
25°C |
 |
| 180 |
 |
|
3455 |
 Nalidixic acid Nalidixic acid
|
 |
 |
mg |
25°C |
 |
| 180 |
 |
|
3455 |
 Nalmefene Nalmefene
|
 |
 |
10 µg/ml |
21°C |
 |
| 72 |
 |
|
1477 |
 Nalmefene Nalmefene
|
 |
 |
10 µg/ml |
40°C |
 |
| 72 |
 |
|
1477 |
 Nalmefene Nalmefene
|
 |
 |
10 µg/ml |
21°C |
 |
| 72 |
 |
|
1477 |
 Nalmefene Nalmefene
|
 |
 |
10 µg/ml |
40°C |
 |
| 72 |
 |
|
1477 |
 Nalmefene Nalmefene
|
 |
 |
10 µg/ml |
21°C |
 |
| 72 |
 |
|
1477 |
 Nalmefene Nalmefene
|
 |
 |
10 µg/ml |
40°C |
 |
| 72 |
 |
|
1477 |
 Naltrexone Naltrexone
|
 |
 |
mg |
25°C |
 |
| 30 |
 |
|
2542 |
 Naltrexone Naltrexone
|
 |
 |
mg |
25°C |
 |
| 30 |
 |
|
2542 |
 Natalizumab Natalizumab
|
 |
 |
2,6 mg/mL |
2-8°C |
 |
| 8 |
 |
|
4068 |
 Netilmicin sulfate Netilmicin sulfate
|
 |
 |
3 mg/ml |
25°C |
 |
| 7 |
 |
|
1200 |
 Netilmicin sulfate Netilmicin sulfate
|
 |
 |
3 mg/ml |
25°C |
 |
| 7 |
 |
|
1200 |
 Netilmicin sulfate Netilmicin sulfate
|
 |
 |
3 mg/ml |
25°C |
 |
| 7 |
 |
|
1200 |
 Netilmicin sulfate Netilmicin sulfate
|
 |
 |
3 mg/ml |
25°C |
 |
| 7 |
 |
|
1200 |
 Netilmicin sulfate Netilmicin sulfate
|
 |
 |
3 mg/ml |
25°C |
 |
| 7 |
 |
|
1200 |
 Nifedipine Nifedipine
|
 |
 |
mgml |
2-8°C |
 |
| 92 |
 |
|
3810 |
 Nifedipine Nifedipine
|
 |
 |
mgml |
25°C |
 |
| 92 |
 |
|
3810 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,05 mg/ml |
25°C |
 |
| 48 |
 |
|
1223 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,656 mg/ml |
25°C |
 |
| 24 |
 |
|
1270 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,05 mg/ml |
25°C |
 |
| 48 |
 |
|
1223 |
 Nivolumab Nivolumab
|
 |
 |
10 mg/ml |
40°C |
 |
| 7 |
 |
|
4505 |
 Nivolumab Nivolumab
|
 |
 |
10 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4505 |
 Nivolumab Nivolumab
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4505 |
 Nizatidine Nizatidine
|
 |
|
25 mg/ml |
15-30°C |
 |
| 8 |
 |
|
2500 |
 Nizatidine Nizatidine
|
 |
|
25 mg/ml |
5°C |
 |
| 48 |
 |
|
2500 |
 Nizatidine Nizatidine
|
 |
|
25 mg/ml |
5°C |
 |
| 48 |
 |
|
2500 |
 Nizatidine Nizatidine
|
 |
|
25 mg/ml |
15-30°C |
 |
| 8 |
 |
|
2500 |
 Nizatidine Nizatidine
|
 |
|
25 mg/ml |
15-30°C |
 |
| 8 |
 |
|
2500 |
 Nizatidine Nizatidine
|
 |
|
25 mg/ml |
5°C |
 |
| 48 |
 |
|
2500 |
 Obinutuzumab Obinutuzumab
|
 |
 |
0,4 >> 20 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3768 |
 Obinutuzumab Obinutuzumab
|
 |
 |
0,4 >> 20 mg/ml |
<30°C |
 |
| 48 |
 |
|
3768 |
 Obinutuzumab Obinutuzumab
|
 |
 |
0,4 >> 20 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3768 |
 Obinutuzumab Obinutuzumab
|
 |
 |
0,4 >> 20 mg/ml |
<30°C |
 |
| 48 |
 |
|
3768 |
 Ofatumumab Ofatumumab
|
 |
 |
0,3 mg/ml |
<25°C |
 |
| 48 |
 |
|
3138 |
 Ofatumumab Ofatumumab
|
 |
 |
2 mg/ml |
<25°C |
 |
| 48 |
 |
|
3138 |
 Omadacycline Omadacycline
|
 |
 |
1 mg/mL |
2-8°C |
 |
| 9 |
 |
|
4719 |
 Omadacycline Omadacycline
|
 |
 |
1 & 2 mg/mL |
2-8°C |
 |
| 7 |
 |
|
4720 |
 Omadacycline Omadacycline
|
 |
 |
1 & 2 mg/mL |
25°C |
 |
| 24 |
 |
|
4720 |
 Omeprazole sodium Omeprazole sodium
|
 |
 |
mg/ml |
24°C |
 |
| 14 |
 |
|
2430 |
 Omeprazole sodium Omeprazole sodium
|
 |
 |
mg/ml |
2°C |
 |
| 56 |
 |
|
2886 |
 Omeprazole sodium Omeprazole sodium
|
 |
 |
mg/ml |
5°C |
 |
| 30 |
 |
|
2430 |
 Omeprazole sodium Omeprazole sodium
|
 |
 |
0.4 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3658 |
 Ondansetron hydrochloride Ondansetron hydrochloride
|
 |
 |
0,24 mg/ml |
30°C |
 |
| 24 |
 |
|
179 |
 Ondansetron hydrochloride Ondansetron hydrochloride
|
 |
 |
2 mg/ml |
30°C |
 |
| 7 |
 |
|
179 |
 Oritavancin Oritavancin
|
 |
 |
1,2 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3787 |
 Oritavancin Oritavancin
|
 |
 |
1,2 mg/ml |
25°C |
 |
| 12 |
 |
|
3787 |
 Oseltamivir Oseltamivir
|
 |
 |
mg/ml |
2-8°C |
 |
| 30 |
 |
|
3090 |
 Oseltamivir Oseltamivir
|
 |
 |
mg/ml |
2-8°C |
 |
| 30 |
 |
|
3090 |
 Oseltamivir Oseltamivir
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
120 mg/ml |
25°C |
 |
| 3 |
 |
|
13 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
10 & 80 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
10 & 100 mg/ml |
25°C |
 |
| 4 |
 |
|
3589 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
10 & 100 mg/ml |
4°C |
 |
| 7 |
 |
|
3589 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
10 & 100 mg/ml |
25°C |
 |
| 4 |
 |
|
3589 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
10 & 100 mg/ml |
4°C |
 |
| 7 |
 |
|
3589 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
0,5 & 2 mg/ml |
25°C |
 |
| 6 |
 |
|
3589 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
10 & 30 mg/ml |
4°C |
 |
| 4 |
 |
|
3589 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
0,5 & 2 mg/ml |
25°C |
 |
| 6 |
 |
|
3589 |
 Oxacillin sodium Oxacillin sodium
|
 |
 |
10 & 30 mg/ml |
4°C |
 |
| 4 |
 |
|
3589 |
 Oxaliplatin Oxaliplatin
|
 |
 |
0,1 mg/ml |
42°C |
 |
| 2 |
 |
|
2791 |
 Oxaliplatin Oxaliplatin
|
 |
 |
0,1 mg/ml |
42°C |
 |
| 0.5 |
 |
|
2791 |
 Oxaliplatin Oxaliplatin
|
 |
 |
0,1 mg/ml |
42°C |
 |
| 0.5 |
 |
|
2791 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
10 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
50 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
50 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
10 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
50 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
50 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
1 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
10 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
50 mg/ml |
25°C |
 |
| 7 |
 |
|
2199 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
50 mg/ml |
37°C |
 |
| 7 |
 |
|
2199 |
 Oxytocin Oxytocin
|
 |
 |
0,02 UI/ml |
4°C |
 |
| 7 |
 |
|
1903 |
 Oxytocin Oxytocin
|
 |
 |
0,02 UI/ml |
4°C |
 |
| 24 |
 |
|
1903 |
 Oxytocin Oxytocin
|
 |
 |
0,02 >> 0,06 UI/ml |
25°C |
 |
| 32 |
 |
|
2165 |
 Oxytocin Oxytocin
|
 |
 |
0,02 >> 0,06 UI/ml |
4°C |
 |
| 32 |
 |
|
2165 |
 Paclitaxel Paclitaxel
|
 |
 |
0,4 & 1,2 mg/ml |
4°C |
 |
| 5 |
 |
|
1520 |
 Paclitaxel Paclitaxel
|
 |
 |
0,1 & 1 mg/ml |
22°C |
 |
| 3 |
 |
|
157 |
 Paclitaxel Paclitaxel
|
 |
 |
0,1 & 1 mg/ml |
32°C |
 |
| 3 |
 |
|
157 |
 Paclitaxel Paclitaxel
|
 |
 |
0,6 mg/ml |
20°C-23°C |
 |
| 25 |
 |
|
412 |
 Paclitaxel Paclitaxel
|
 |
 |
1,2 mg/ml |
20°C-23°C |
 |
| 25 |
 |
|
412 |
 Paclitaxel Paclitaxel
|
 |
 |
1,2 mg/ml |
20°C-23°C |
 |
| 12 |
 |
|
412 |
 Paclitaxel albumin Paclitaxel albumin
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3157 |
 Paclitaxel albumin Paclitaxel albumin
|
 |
 |
5 mg/ml |
25°C |
 |
| 4 |
 |
|
3157 |
 Paclitaxel albumin Paclitaxel albumin
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3157 |
 Paclitaxel albumin Paclitaxel albumin
|
 |
 |
5 mg/ml |
25°C |
 |
| 4 |
 |
|
3157 |
 Palonosetron hydrochloride Palonosetron hydrochloride
|
 |
 |
25 µg/ml |
25°C |
 |
| 4 |
 |
|
2281 |
 Pancuronium bromide Pancuronium bromide
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 912 |
 |
|
4773 |
 Panitumumab Panitumumab
|
 |
 |
<10 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4807 |
 Panitumumab Panitumumab
|
 |
 |
<10 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4807 |
 Paracetamol Paracetamol
|
 |
 |
10 mg/ml |
23-25°C |
 |
| 84 |
 |
|
3350 |
 Paracetamol Paracetamol
|
 |
 |
> 1 mg/ml mg/ml |
23°C |
 |
| 48 |
 |
|
3769 |
 Pegaspargase Pegaspargase
|
 |
 |
750 UI |
< 25°C |
 |
| 24 |
 |
|
4173 |
 Pegaspargase Pegaspargase
|
 |
 |
? UI |
2-8°C |
 |
| 48 |
 |
|
4173 |
 Pembrolizumab Pembrolizumab
|
 |
 |
25 mg/ml |
25°C |
 |
| 24 |
 |
|
3771 |
 Pembrolizumab Pembrolizumab
|
 |
 |
1 mg/ml |
4°C |
 |
| 14 |
 |
|
4786 |
 Pembrolizumab Pembrolizumab
|
 |
 |
4 mg/ml |
25°C |
 |
| 7 |
 |
|
4786 |
 Pembrolizumab Pembrolizumab
|
 |
 |
4 mg/ml |
4°C |
 |
| 30 |
 |
|
4786 |
 Pembrolizumab Pembrolizumab
|
 |
 |
1 >> 10 mg/ml |
2-8°C |
 |
| 96 |
 |
|
3771 |
 Pemetrexed disodium Pemetrexed disodium
|
 |
 |
25 mg/ml |
2-8°C |
 |
| 24 |
 |
|
1914 |
 Penicillin G potassium Penicillin G potassium
|
 |
 |
0,1 & 0,2 MUI/ml |
25°C |
 |
| 3 |
 |
|
13 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0,1 & 0,2 MUI/ml |
25°C |
 |
| 3 |
 |
|
13 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0.0025 MUI/ml |
5°C |
 |
| 21 |
 |
|
3648 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0.05 MUI/ml |
5°C |
 |
| 21 |
 |
|
3648 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0.0025 MUI/ml |
5°C |
 |
| 21 |
 |
|
3648 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0.05 MUI/ml |
5°C |
 |
| 21 |
 |
|
3648 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0,13 MUI/ml |
21°C-22°C |
 |
| 13 |
 |
|
2056 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0,13 MUI/ml |
26°C |
 |
| 12 |
 |
|
2056 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0,13 MUI/ml |
3-5°C |
 |
| 6 |
 |
|
2056 |
 Penicillin G sodium Penicillin G sodium
|
 |
 |
0,13 MUI/ml |
36°C |
 |
| 5 |
 |
|
2056 |
 Pentamidine isetionate Pentamidine isetionate
|
 |
 |
2 mg/ml |
20°C |
 |
| 24 |
 |
|
1235 |
 Pentamidine isetionate Pentamidine isetionate
|
 |
 |
2 & 6 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 31 |
 |
|
1628 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 31 |
 |
|
1628 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
50 mg/ml |
25°C |
 |
| 100 |
 |
|
4303 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
mg/ml |
22°C |
 |
| 21 |
 |
|
3845 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
mg/ml |
22°C |
 |
| 14 |
 |
|
3845 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
mg/ml |
22°C |
 |
| 60 |
 |
|
3845 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
mg/ml |
4°C |
 |
| 60 |
 |
|
3845 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
mg/ml |
4°C |
 |
| 14 |
 |
|
3845 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
mg/ml |
4°C |
 |
| 60 |
 |
|
3845 |
 Pentobarbital sodium Pentobarbital sodium
|
 |
 |
mg/ml |
4°C |
 |
| 30 |
 |
|
3845 |
 Pentostatin Pentostatin
|
 |
 |
0.002 >> 0.02 mg/ml |
22°C-23°C |
 |
| 48 |
 |
|
154 |
 Pentostatin Pentostatin
|
 |
 |
2 mg/ml |
22°C-23°C |
 |
| 72 |
 |
|
154 |
 Pentostatin Pentostatin
|
 |
 |
0.002 >> 0.02 mg/ml |
22°C-23°C |
 |
| 48 |
 |
|
154 |
 Pertuzumab Pertuzumab
|
 |
 |
3 mg/ml |
5°C |
 |
| 24 |
 |
|
3366 |
 Pertuzumab Pertuzumab
|
 |
 |
3 mg/ml |
5°C |
 |
| 24 |
 |
|
3366 |
 Pethidine hydrochloride Pethidine hydrochloride
|
 |
 |
10 mg/ml |
37°C |
 |
| 90 |
 |
|
1735 |
 Phenobarbital sodium Phenobarbital sodium
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Phenobarbital sodium Phenobarbital sodium
|
 |
 |
mg/ml |
25°C |
 |
| 154 |
 |
|
3810 |
 Phenobarbital sodium Phenobarbital sodium
|
 |
 |
mg/ml |
25°C |
 |
| 154 |
 |
|
3810 |
 Phenylbutyrate sodium Phenylbutyrate sodium
|
 |
 |
mg |
23-25°C |
 |
| 90 |
 |
|
2399 |
 Phenylbutyrate sodium Phenylbutyrate sodium
|
 |
 |
mg |
23-25°C |
 |
| 90 |
 |
|
2399 |
 Phenylephrine hydrochloride Phenylephrine hydrochloride
|
 |
 |
0,1 & 0,2 mg/ml |
25°C |
 |
| 14 |
 |
|
1891 |
 Phenylephrine hydrochloride Phenylephrine hydrochloride
|
 |
 |
0,02 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3770 |
 Phenylephrine hydrochloride Phenylephrine hydrochloride
|
 |
 |
0,02 mg/ml |
25°C |
 |
| 4 |
 |
|
3770 |
 Phenylephrine hydrochloride Phenylephrine hydrochloride
|
 |
 |
0,1 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3770 |
 Phenylephrine hydrochloride Phenylephrine hydrochloride
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 4 |
 |
|
3770 |
 Phenytoin sodium Phenytoin sodium
|
 |
 |
9,2 & 18,4 mg/ml |
25°C |
 |
| 2 |
 |
|
310 |
 Pimavanserin Pimavanserin
|
 |
 |
mg/mL |
25°C |
 |
| 24 |
 |
|
4484 |
 Pimavanserin Pimavanserin
|
 |
 |
mg/mL |
25°C |
 |
| 24 |
 |
|
4484 |
 Pimavanserin Pimavanserin
|
 |
 |
mg/mL |
25°C |
 |
| 24 |
 |
|
4484 |
 Piperacillin sodium Piperacillin sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 120 |
 |
|
600 |
 Piperacillin sodium Piperacillin sodium
|
 |
 |
40 mg/ml |
25°C |
 |
| 5 |
 |
|
1718 |
 Piperacillin sodium Piperacillin sodium
|
 |
 |
10 & 80 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Piperacillin sodium Piperacillin sodium
|
 |
 |
128 mg/ml |
25°C |
 |
| 30 |
 |
|
2042 |
 Piperacillin sodium Piperacillin sodium
|
 |
 |
128 mg/ml |
37°C |
 |
| 21 |
 |
|
2042 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
80 / 10 mg/ml |
25°C |
 |
| 24 |
 |
|
274 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
33.3 / 4.1 mg/ml |
4°C |
 |
| 35 |
 |
|
1946 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
50.6 / 6.3 mg/ml |
2-8°C |
 |
| 17 |
 |
|
4620 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
50.6 / 6.3 mg/ml |
25°C |
 |
| 3 |
 |
|
4620 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
33,3 / 4,1 mg/ml |
2-8°C |
 |
| 58 |
 |
|
4148 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
50.6 / 6.3 mg/ml |
2-8°C |
 |
| 17 |
 |
|
4621 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
50.6 / 6.3 mg/ml |
25°C |
 |
| 3 |
 |
|
4621 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
150 / 18.75 mg/ml |
25°C |
 |
| 24 |
 |
|
274 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
200 / 25 mg/ml |
25°C |
 |
| 24 |
 |
|
274 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
80 / 10 & 10 / 1.25 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
22 / 3 mg/ml |
2-8°C |
 |
| 13 |
 |
|
4512 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
22 / 3 mg/ml |
32°C |
 |
| 24 |
 |
|
4512 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
80 / 10 mg/ml |
2-8°C |
 |
| 13 |
 |
|
4512 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
80 / 10 mg/ml |
32°C |
 |
| 24 |
 |
|
4512 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
22 / 3 mg/ml |
2-8°C |
 |
| 13 |
 |
|
4512 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
22 / 3 mg/ml |
32°C |
 |
| 24 |
 |
|
4512 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
80 / 10 mg/ml |
2-8°C |
 |
| 13 |
 |
|
4512 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
80 / 10 mg/ml |
32°C |
 |
| 24 |
 |
|
4512 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
128 / 16 mg/ml |
25°C |
 |
| 72 |
 |
|
2042 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
128 / 16 mg/ml |
37°C |
 |
| 24 |
 |
|
2042 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
0.2 / 0.025 mg/ml |
23°C |
 |
| 7 |
 |
|
2451 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
0.2 / 0.025 mg/ml |
37°C |
 |
| 24 |
 |
|
2451 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
0.2 / 0.025 mg/ml |
4°C |
 |
| 14 |
 |
|
2451 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
0.2 / 0.025 mg/ml |
23°C |
 |
| 7 |
 |
|
2451 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
0.2 / 0.025 mg/ml |
37°C |
 |
| 24 |
 |
|
2451 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
0.2 / 0.025 mg/ml |
4°C |
 |
| 14 |
 |
|
2451 |
 Piritramide Piritramide
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 28 |
 |
|
1697 |
 Piritramide Piritramide
|
 |
 |
1 mg/ml |
25°C |
 |
| 28 |
 |
|
1697 |
 Plazomicin sulfate Plazomicin sulfate
|
 |
 |
2.5 >> 45 mg/ml |
2-8°C |
 |
| 7 |
 |
|
4482 |
 Plazomicin sulfate Plazomicin sulfate
|
 |
 |
2.5 >> 45 mg/ml |
25°C |
 |
| 24 |
 |
|
4482 |
 Plazomicin sulfate Plazomicin sulfate
|
 |
 |
2.5 >> 45 mg/ml |
2-8°C |
 |
| 7 |
 |
|
4482 |
 Plazomicin sulfate Plazomicin sulfate
|
 |
 |
2.5 >> 45 mg/ml |
25°C |
 |
| 24 |
 |
|
4482 |
 Polymyxine B Polymyxine B
|
 |
 |
2 mg/ml |
25-30°C |
 |
| 24 |
 |
|
3895 |
 Polymyxine B Polymyxine B
|
 |
 |
2 mg/ml |
4°C |
 |
| 168 |
 |
|
3895 |
 Polymyxine B Polymyxine B
|
 |
 |
2 mg/ml |
25-30°C |
 |
| 24 |
 |
|
3895 |
 Polymyxine B Polymyxine B
|
 |
 |
2 mg/ml |
4°C |
 |
| 168 |
 |
|
3895 |
 Polymyxine B Polymyxine B
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 24 |
 |
|
3121 |
 Polymyxine B Polymyxine B
|
 |
 |
0,1 mg/ml |
4°C |
 |
| 24 |
 |
|
3121 |
 Posaconazole Posaconazole
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4380 |
 Posaconazole Posaconazole
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4380 |
 Posaconazole Posaconazole
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4380 |
 Posaconazole Posaconazole
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4380 |
 Posaconazole Posaconazole
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4380 |
 Posaconazole Posaconazole
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4380 |
 Pravastatine Pravastatine
|
 |
 |
|
2-8°C |
 |
| 60 |
 |
|
3898 |
 Pravastatine Pravastatine
|
 |
 |
|
22-25°C |
 |
| 60 |
 |
|
3898 |
 Prednisone Prednisone
|
 |
 |
mg/ml |
2-8°C |
 |
| 30 |
 |
|
3810 |
 Prednisone Prednisone
|
 |
 |
mg/ml |
2-8°C |
 |
| 30 |
 |
|
3810 |
 Prednisone Prednisone
|
 |
 |
mg/ml |
25°C |
 |
| 30 |
 |
|
3810 |
 Prednisone Prednisone
|
 |
 |
mg/ml |
25°C |
 |
| 30 |
 |
|
3810 |
 Procainamide hydrochloride Procainamide hydrochloride
|
 |
 |
4 mg/ml |
25°C |
 |
| 48 |
 |
|
1115 |
 Procainamide hydrochloride Procainamide hydrochloride
|
 |
 |
4 & 8 mg/ml |
23-25°C |
 |
| 6 |
 |
|
370 |
 Propafenone Hydrochloride Propafenone Hydrochloride
|
 |
 |
mg/mL |
2-8°C |
 |
| 90 |
 |
|
4547 |
 Propafenone Hydrochloride Propafenone Hydrochloride
|
 |
 |
mg/mL |
22-30°C |
 |
| 90 |
 |
|
4547 |
 Propofol Propofol
|
 |
 |
< 2 mg/ml |
25°C |
 |
| 6 |
 |
|
3659 |
 Propofol Propofol
|
 |
 |
< 2 mg/ml |
25°C |
 |
| 6 |
 |
|
3659 |
 Propofol Propofol
|
 |
 |
< 2 mg/ml |
25°C |
 |
| 6 |
 |
|
3659 |
 Propranolol hydrochloride Propranolol hydrochloride
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Pyrazinamide Pyrazinamide
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Pyrazinamide Pyrazinamide
|
 |
 |
mg/ml |
20-25°C |
 |
| 90 |
 |
|
4177 |
 Pyrazinamide Pyrazinamide
|
 |
 |
mg/ml |
25°C |
 |
| 60 |
 |
|
2454 |
 Pyrazinamide Pyrazinamide
|
 |
 |
mg/ml |
4°C |
 |
| 60 |
 |
|
2454 |
 Quinupristine/dalfopristine Quinupristine/dalfopristine
|
 |
 |
? mg/ml |
25°C |
 |
| 5 |
 |
|
1513 |
 Ramucirumab Ramucirumab
|
 |
 |
mg/ml |
2-8°C |
 |
| 24 |
 |
|
3773 |
 Ramucirumab Ramucirumab
|
 |
 |
mg/ml |
25°C |
 |
| 4 |
 |
|
3773 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 15 |
 |
|
693 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
2,5 mg/ml |
22°C |
 |
| 72 |
 |
|
46 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
mg/ml |
2-8°C |
 |
| 58 |
 |
|
3810 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 36 |
 |
|
3810 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
10 >> 50 µg/ml |
20-22°C |
 |
| 24 |
 |
|
4501 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
10 >> 50 µg/ml |
20-22°C |
 |
| 24 |
 |
|
4501 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
10 µg/ml |
2-8°C |
 |
| 21 |
 |
|
4192 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
10 µg/ml |
23-27°C |
 |
| 90 |
 |
|
4183 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
20 µg/ml |
2-8°C |
 |
| 21 |
 |
|
4192 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
4 µg/ml |
23-27°C |
 |
| 90 |
 |
|
4183 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
4 & 10 µg/ml |
23°C |
 |
| 90 |
 |
|
4231 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
50 µg/ml |
2-8°C |
 |
| 21 |
 |
|
4192 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
20 >> 250 µg/ml |
25°C |
 |
| 24 |
 |
|
3519 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
20 >> 250 µg/ml |
25°C |
 |
| 24 |
 |
|
3519 |
 Remifentanil hydrochloride Remifentanil hydrochloride
|
 |
 |
20 >> 250 µg/ml |
25°C |
 |
| 24 |
 |
|
3519 |
 Reteplase Reteplase
|
 |
 |
1 UI/ml |
2-30°C |
 |
| 8 |
 |
|
2212 |
 Retinol Retinol
|
 |
 |
mg |
25°C |
 |
| 60 |
 |
|
2988 |
 Ribavirine Ribavirine
|
 |
 |
mg/ml |
4°C |
 |
| 21 |
 |
|
2852 |
 Rifabutin Rifabutin
|
 |
 |
mg/ml |
25°C |
 |
| 84 |
 |
|
2419 |
 Rifabutin Rifabutin
|
 |
 |
mg/ml |
25°C |
 |
| 84 |
 |
|
2419 |
 Rifabutin Rifabutin
|
 |
 |
mg/ml |
30-40°C |
 |
| 56 |
 |
|
2419 |
 Rifabutin Rifabutin
|
 |
 |
mg/ml |
30-40°C |
 |
| 84 |
 |
|
2419 |
 Rifabutin Rifabutin
|
 |
 |
mg/ml |
4°C |
 |
| 84 |
 |
|
2419 |
 Rifabutin Rifabutin
|
 |
 |
mg/ml |
4°C |
 |
| 84 |
 |
|
2419 |
 Rifampicin Rifampicin
|
 |
 |
0,1 mg/ml |
24°C |
 |
| 8 |
 |
|
263 |
 Rifampicin Rifampicin
|
 |
 |
0,1 mg/ml |
4°C |
 |
| 72 |
 |
|
263 |
 Rifampicin Rifampicin
|
 |
 |
mg |
21-23°C |
 |
| 56 |
 |
|
2704 |
 Rifampicin Rifampicin
|
 |
 |
mg |
21-23°C |
 |
| 42 |
 |
|
2704 |
 Rituximab Rituximab
|
 |
 |
10 mg/ml |
23-32°C |
 |
| 21 |
 |
|
4708 |
 Rituximab Rituximab
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 31 |
 |
|
4040 |
 Rituximab Rituximab
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 31 |
 |
|
4040 |
 Rituximab Rituximab
|
 |
 |
120 mg/ml |
2-8°C |
 |
| 28 |
 |
|
2825 |
 Rituximab Rituximab
|
 |
 |
1 >> 4 mg/ml |
2-8°C |
 |
| 30 |
 |
|
4740 |
 Rituximab Rituximab
|
 |
 |
1 >> 4 mg/ml |
<30°C |
 |
| 24 |
 |
|
4740 |
 Rituximab Rituximab
|
 |
 |
1 >> 4 mg/ml |
2-8°C |
 |
| 24 |
 |
|
4740 |
 Rituximab Rituximab
|
 |
 |
1 >> 4 mg/ml |
25°C |
 |
| 12 |
 |
|
4740 |
 Romidepsin Romidepsin
|
 |
 |
0.05 mg/ml |
25°C |
 |
| 24 |
 |
|
3432 |
 Romidepsin Romidepsin
|
 |
 |
5 mg/ml |
25°C |
 |
| 8 |
 |
|
3432 |
 Romidepsin Romidepsin
|
 |
 |
0.05 mg/ml |
25°C |
 |
| 24 |
 |
|
3432 |
 Romidepsin Romidepsin
|
 |
 |
0.05 mg/ml |
25°C |
 |
| 24 |
 |
|
3432 |
 Romidepsin Romidepsin
|
 |
 |
0.05 mg/ml |
25°C |
 |
| 24 |
 |
|
3432 |
 Salbutamol sulfate Salbutamol sulfate
|
 |
 |
mg/ml |
22-26 °C |
 |
| 7 |
 |
|
2670 |
 Salbutamol sulfate Salbutamol sulfate
|
 |
 |
mg/ml |
4°C |
 |
| 7 |
 |
|
2670 |
 Salbutamol sulfate Salbutamol sulfate
|
 |
 |
mg/ml |
22-26°C |
 |
| 7 |
 |
|
2670 |
 Salbutamol sulfate Salbutamol sulfate
|
 |
 |
mg/ml |
4°C |
 |
| 7 |
 |
|
2670 |
 Salbutamol sulfate Salbutamol sulfate
|
 |
 |
mg/ml |
22-26°C |
 |
| 7 |
 |
|
2670 |
 Salbutamol sulfate Salbutamol sulfate
|
 |
 |
mg/ml |
4°C |
 |
| 7 |
 |
|
2670 |
 Salbutamol sulfate Salbutamol sulfate
|
 |
 |
mg/ml |
22-26°C |
 |
| 7 |
 |
|
2670 |
 Salbutamol sulfate Salbutamol sulfate
|
 |
 |
mg/ml |
4°C |
 |
| 7 |
 |
|
2670 |
 Sildenafil citrate Sildenafil citrate
|
 |
 |
0,4 mg/ml |
5°C |
 |
| 30 |
 |
|
3923 |
 Sildenafil citrate Sildenafil citrate
|
 |
 |
0,067 mg/ml |
25°C |
 |
| 7 |
 |
|
3274 |
 Sildenafil citrate Sildenafil citrate
|
 |
 |
0,067 mg/ml |
37°C |
 |
| 7 |
 |
|
3274 |
 Sildenafil citrate Sildenafil citrate
|
 |
 |
0,667 mg/ml |
25°C |
 |
| 7 |
 |
|
3274 |
 Sildenafil citrate Sildenafil citrate
|
 |
 |
0,667 mg/ml |
37°C |
 |
| 7 |
 |
|
3274 |
 Siltuximab Siltuximab
|
 |
 |
20 mg/ml |
25°C |
 |
| 2 |
 |
|
3772 |
 Siltuximab Siltuximab
|
 |
 |
mg/ml |
25°C |
 |
| 6 |
 |
|
3772 |
 Siltuximab Siltuximab
|
 |
 |
mg/ml |
25°C |
 |
| 6 |
 |
|
3772 |
 Siltuximab Siltuximab
|
 |
 |
mg/ml |
25°C |
 |
| 6 |
 |
|
3772 |
 Siltuximab Siltuximab
|
 |
 |
mg/ml |
25°C |
 |
| 6 |
 |
|
3772 |
 Simvastatin Simvastatin
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
3810 |
 Sitagliptine phosphate Sitagliptine phosphate
|
 |
 |
mg/mL |
22-28°C |
 |
| 40 |
 |
|
4679 |
 Sitagliptine phosphate Sitagliptine phosphate
|
 |
 |
mg/mL |
40°C |
 |
| 40 |
 |
|
4679 |
 Sodium bicarbonate Sodium bicarbonate
|
 |
 |
4 >> 11,45 mg/ml |
2-4°C |
 |
| 7 |
 |
|
3116 |
 Sodium bicarbonate Sodium bicarbonate
|
 |
 |
0,336 mg/ml |
25°C |
 |
| 48 |
 |
|
4383 |
 Sodium bicarbonate Sodium bicarbonate
|
 |
 |
4 >> 11,45 mg/ml |
2-4°C |
 |
| 7 |
 |
|
3116 |
 Sodium citrate Sodium citrate
|
 |
 |
40 mg/ml |
37°C |
 |
| 96 |
 |
|
3952 |
 Sodium ferric gluconate complex with sucrose Sodium ferric gluconate complex with sucrose
|
 |
 |
0,625 mg/ml |
18-25°C |
 |
| 24 |
 |
|
3456 |
 Sodium ferric gluconate complex with sucrose Sodium ferric gluconate complex with sucrose
|
 |
 |
0,625 mg/ml |
2-8°C |
 |
| 7 |
 |
|
3456 |
 Sodium ferric gluconate complex with sucrose Sodium ferric gluconate complex with sucrose
|
 |
 |
1,25 mg/ml |
18-25°C |
 |
| 24 |
 |
|
3456 |
 Sodium ferric gluconate complex with sucrose Sodium ferric gluconate complex with sucrose
|
 |
 |
1,25 mg/ml |
2-8°C |
 |
| 7 |
 |
|
3456 |
 Sodium ferric gluconate complex with sucrose Sodium ferric gluconate complex with sucrose
|
 |
 |
12,5 mg/ml |
18-25°C |
 |
| 48 |
 |
|
3456 |
 Sodium ferric gluconate complex with sucrose Sodium ferric gluconate complex with sucrose
|
 |
 |
12,5 mg/ml |
2-8°C |
 |
| 7 |
 |
|
3456 |
 Sotalol hydrochloride Sotalol hydrochloride
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Sotalol hydrochloride Sotalol hydrochloride
|
 |
 |
mg/ml |
20-25°C |
 |
| 90 |
 |
|
4177 |
 Spiramycine adipate Spiramycine adipate
|
 |
 |
0.375 MUI/ml |
? |
 |
| 12 |
 |
|
3563 |
 Spironolactone Spironolactone
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Spironolactone Spironolactone
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Spironolactone Spironolactone
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
3810 |
 Spironolactone Spironolactone
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
3810 |
 Streptokinase Streptokinase
|
 |
 |
360000 UI/ml |
2-8°C |
 |
| 24 |
 |
|
3625 |
 Streptozocin Streptozocin
|
 |
 |
? mg/ml |
25°C |
 |
| 24 |
 |
|
3654 |
 Sufentanil citrate Sufentanil citrate
|
 |
 |
0,005 mg/ml |
32°C |
 |
| 5 |
 |
|
675 |
 Sufentanil citrate Sufentanil citrate
|
 |
 |
0,005 mg/ml |
32°C |
 |
| 3 |
 |
|
510 |
 Sufentanil citrate Sufentanil citrate
|
 |
 |
0,05 mg/ml |
25°C |
 |
| 14 |
 |
|
2118 |
 Sufentanil citrate Sufentanil citrate
|
 |
 |
0,005 mg/ml |
32°C |
 |
| 5 |
 |
|
675 |
 Sugammadex Sugammadex
|
 |
 |
10 mg/ml |
2-8°C |
 |
| 48 |
 |
|
2328 |
 Sugammadex Sugammadex
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
2328 |
 Suxamethonium chloride Suxamethonium chloride
|
 |
 |
50 mg/ml |
22-26°C |
 |
| 17 |
 |
|
4274 |
 Tacrolimus Tacrolimus
|
 |
 |
4 >>> 100 µg/ml |
2-8°C |
 |
| 24 |
 |
|
3626 |
 Tacrolimus Tacrolimus
|
 |
 |
4 >>> 100 µg/ml |
25°C |
 |
| 24 |
 |
|
3626 |
 Tacrolimus Tacrolimus
|
 |
 |
4 >>> 100 µg/ml |
2-8°C |
 |
| 24 |
 |
|
3626 |
 Tacrolimus Tacrolimus
|
 |
 |
4 >>> 100 µg/ml |
25°C |
 |
| 24 |
 |
|
3626 |
 Tacrolimus Tacrolimus
|
 |
 |
4 >>> 100 µg/ml |
2-8°C |
 |
| 24 |
 |
|
3626 |
 Tacrolimus Tacrolimus
|
 |
 |
4 >>> 100 µg/ml |
25°C |
 |
| 24 |
 |
|
3626 |
 Tacrolimus Tacrolimus
|
 |
 |
10 µg/ml |
20-25°C |
 |
| 48 |
 |
|
3842 |
 Tacrolimus Tacrolimus
|
 |
 |
100 µg/ml |
20-25°C |
 |
| 48 |
 |
|
3842 |
 Tacrolimus Tacrolimus
|
 |
 |
1 µg/ml |
20-25°C |
 |
| 24 |
 |
|
3842 |
 Tacrolimus Tacrolimus
|
 |
 |
1 µg/ml |
20-25°C |
 |
| 48 |
 |
|
3842 |
 Tacrolimus Tacrolimus
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Tacrolimus Tacrolimus
|
 |
 |
mg/ml |
2-8°C |
 |
| 270 |
 |
|
4673 |
 Tacrolimus Tacrolimus
|
 |
 |
mg/ml |
2-8°C |
 |
| 270 |
 |
|
4673 |
 Tedizolid phosphate Tedizolid phosphate
|
 |
 |
50 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3753 |
 Tedizolid phosphate Tedizolid phosphate
|
 |
 |
50 mg/ml |
25°C |
 |
| 24 |
 |
|
3753 |
 Tedizolid phosphate Tedizolid phosphate
|
 |
 |
0,8 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3753 |
 Tedizolid phosphate Tedizolid phosphate
|
 |
 |
0,8 mg/ml |
25°C |
 |
| 24 |
 |
|
3753 |
 Teicoplanine Teicoplanine
|
 |
 |
133 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3656 |
 Teicoplanine Teicoplanine
|
 |
 |
? mg/ml |
2-8°C |
 |
| 24 |
 |
|
3656 |
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
0,6 >> 8 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3260 |
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
0,6 >> 8 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3260 |
 Temocilline Temocilline
|
 |
 |
125 mg/ml |
25°C |
 |
| 24 |
 |
|
2296 |
 Temocilline Temocilline
|
 |
 |
125 mg/ml |
25°C |
 |
| 24 |
 |
|
2296 |
 Temocilline Temocilline
|
 |
 |
125 mg/ml |
25°C |
 |
| 24 |
 |
|
2296 |
 Temocilline Temocilline
|
 |
 |
125 mg/ml |
25°C |
 |
| 24 |
 |
|
2296 |
 Temocilline Temocilline
|
 |
 |
10 mg/ml |
4°C |
 |
| 28 |
 |
|
3154 |
 Temocilline Temocilline
|
 |
 |
20 mg/ml |
4°C |
 |
| 28 |
 |
|
3154 |
 Temozolomide Temozolomide
|
 |
 |
2,5 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3117 |
 Temozolomide Temozolomide
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 14 |
 |
|
3117 |
 Temozolomide Temozolomide
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 14 |
 |
|
3117 |
 Temozolomide Temozolomide
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 14 |
 |
|
3117 |
 Tenecteplase Tenecteplase
|
 |
 |
1 mg/ml |
37°C |
 |
| 48 |
 |
|
3953 |
 Tenecteplase Tenecteplase
|
 |
 |
1 mg/ml |
37°C |
 |
| 48 |
 |
|
3953 |
 Teniposide Teniposide
|
 |
 |
0,1 mg/ml |
? |
 |
| 5 |
 |
|
504 |
 Teniposide Teniposide
|
 |
 |
0,05 mg/ml |
4°C |
 |
| 8 |
 |
|
1520 |
 Terbinafine Terbinafine
|
 |
 |
mg/ml |
25°C |
 |
| 42 |
 |
|
2417 |
 Terbinafine Terbinafine
|
 |
 |
mg/ml |
4°C |
 |
| 42 |
 |
|
2417 |
 Terbutaline sulfate Terbutaline sulfate
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 23 |
 |
|
1933 |
 Thiopental sodium Thiopental sodium
|
 |
 |
25 mg/ml |
22°C |
 |
| 6 |
 |
|
871 |
 Thiopental sodium Thiopental sodium
|
 |
 |
25 mg/ml |
25°C |
 |
| 5 |
 |
|
527 |
 Thiopental sodium Thiopental sodium
|
 |
 |
25 mg/ml |
3°C |
 |
| 6 |
 |
|
871 |
 Thiotepa Thiotepa
|
 |
 |
10 mg/ml |
2-8°C |
 |
| 8 |
 |
|
3603 |
 Thiotepa Thiotepa
|
 |
 |
10 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4748 |
 Thiotepa Thiotepa
|
 |
 |
1 & 2 & 3 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4748 |
 Thiotepa Thiotepa
|
 |
 |
0.5 & 1 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3603 |
 Thiotepa Thiotepa
|
 |
 |
0.5 & 1 mg/ml |
25°C |
 |
| 4 |
 |
|
3603 |
 Tiagabine Tiagabine
|
 |
 |
mg/ml |
25°C |
 |
| 30 |
 |
|
4198 |
 Tiagabine Tiagabine
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
4198 |
 Ticarcillin sodium Ticarcillin sodium
|
 |
 |
mg/ml |
4°C |
 |
| 7 |
 |
|
2020 |
 Tizanidine hydrochloride Tizanidine hydrochloride
|
 |
 |
mg/mL |
15-30°C |
 |
| 7 |
 |
|
4637 |
 Tizanidine hydrochloride Tizanidine hydrochloride
|
 |
 |
mg/mL |
2-8°C |
 |
| 7 |
 |
|
4637 |
 Tizanidine hydrochloride Tizanidine hydrochloride
|
 |
 |
mg/mL |
40°C |
 |
| 7 |
 |
|
4637 |
 Tizanidine hydrochloride Tizanidine hydrochloride
|
 |
 |
mg/mL |
15-30°C |
 |
| 7 |
 |
|
4637 |
 Tizanidine hydrochloride Tizanidine hydrochloride
|
 |
 |
mg/mL |
2-8°C |
 |
| 7 |
 |
|
4637 |
 Tizanidine hydrochloride Tizanidine hydrochloride
|
 |
 |
mg/mL |
40°C |
 |
| 7 |
 |
|
4637 |
 Tobramycin sulfate Tobramycin sulfate
|
 |
 |
|
25°C |
 |
| 195 |
 |
|
3431 |
 Tobramycin sulfate Tobramycin sulfate
|
 |
 |
|
4°C |
 |
| 195 |
 |
|
3431 |
 Tobramycin sulfate Tobramycin sulfate
|
 |
 |
1 & 10 mg/ml |
25°C |
 |
| 3 |
 |
|
13 |
 Tobramycin sulfate Tobramycin sulfate
|
 |
 |
40 mg/ml |
25°C |
 |
| 60 |
 |
|
709 |
 Tobramycin sulfate Tobramycin sulfate
|
 |
 |
0,5 & 5 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Topotecan Topotecan
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 48 |
 |
|
753 |
 Topotecan Topotecan
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
753 |
 Topotecan Topotecan
|
 |
 |
1 mg/ml |
25°C |
 |
| 28 |
 |
|
1274 |
 Trabectedine Trabectedine
|
 |
 |
0,05 mg/ml |
25°C |
 |
| 30 |
 |
|
2276 |
 Trabectedine Trabectedine
|
 |
 |
≤ 0.030 mg/ml |
25°C |
 |
| 30 |
 |
|
2276 |
 Trabectedine Trabectedine
|
 |
 |
≤ 0.030 mg/ml |
25°C |
 |
| 30 |
 |
|
2276 |
 Tramadol hydrochloride Tramadol hydrochloride
|
 |
 |
1 mg/ml |
4°C |
 |
| 60 |
 |
|
1892 |
 Tramadol hydrochloride Tramadol hydrochloride
|
 |
 |
mg/ml |
23-25°C |
 |
| 90 |
 |
|
2406 |
 Tramadol hydrochloride Tramadol hydrochloride
|
 |
 |
mg/ml |
23-25°C |
 |
| 90 |
 |
|
2406 |
 Tramadol hydrochloride Tramadol hydrochloride
|
 |
 |
mg/ml |
3-5°C |
 |
| 90 |
 |
|
2406 |
 Tramadol hydrochloride Tramadol hydrochloride
|
 |
 |
mg/ml |
3-5°C |
 |
| 90 |
 |
|
2406 |
 Trastuzumab Trastuzumab
|
 |
 |
21 mg/ml |
2-8°C |
 |
| 48 |
 |
|
3077 |
 Trastuzumab Trastuzumab
|
 |
 |
mg/ml |
<30°C |
 |
| 24 |
 |
|
3077 |
 Trastuzumab Trastuzumab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 30 |
 |
|
3077 |
 Trastuzumab Trastuzumab
|
 |
 |
0,2 & 4 mg/ml |
2-8°C |
 |
| 42 |
 |
|
4754 |
 Trastuzumab Trastuzumab
|
 |
 |
0,4 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4194 |
 Trastuzumab Trastuzumab
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4194 |
 Trastuzumab Trastuzumab
|
 |
 |
4 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4194 |
 Trastuzumab Trastuzumab
|
 |
 |
mg/ml |
<30°C |
 |
| 24 |
 |
|
3077 |
 Trastuzumab Trastuzumab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 30 |
 |
|
3077 |
 Trastuzumab Trastuzumab
|
 |
 |
mg/ml |
<30°C |
 |
| 24 |
 |
|
3077 |
 Trastuzumab Trastuzumab
|
 |
 |
? mg/ml |
2-8°C |
 |
| 30 |
 |
|
3077 |
 Trastuzumab Trastuzumab
|
 |
 |
0,4 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4194 |
 Trastuzumab Trastuzumab
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4194 |
 Trastuzumab Trastuzumab
|
 |
 |
4 mg/ml |
2-8°C |
 |
| 28 |
 |
|
4194 |
 Trastuzumab Trastuzumab
|
 |
 |
0,2 & 4 mg/ml |
2-8°C |
 |
| 42 |
 |
|
4754 |
 Trastuzumab Trastuzumab
|
 |
 |
0.8 & 2.4 mg/ml |
25°C |
 |
| 3 |
 |
|
4493 |
 Trastuzumab Trastuzumab
|
 |
 |
0.8 & 2.4 mg/ml |
4°C |
 |
| 90 |
 |
|
4493 |
 Trastuzumab Trastuzumab
|
 |
 |
0,2 & 4 mg/ml |
2-8°C |
 |
| 42 |
 |
|
4754 |
 Trastuzumab Trastuzumab
|
 |
 |
120 mg/ml |
2-8°C |
 |
| 28 |
 |
|
2975 |
 Trastuzumab Trastuzumab
|
 |
 |
120 mg/ml |
2-8°C |
 |
| 28 |
 |
|
2975 |
 Trastuzumab Trastuzumab
|
 |
 |
0.4 >> 4 mg/ml |
2-8°C |
 |
| 28 |
 |
|
3849 |
 Trastuzumab emtansine Trastuzumab emtansine
|
 |
 |
20 mg/ml |
2-8°C |
 |
| 24 |
 |
|
2990 |
 Trastuzumab emtansine Trastuzumab emtansine
|
 |
 |
? mg/ml |
2-8°C |
 |
| 24 |
 |
|
2990 |
 Trastuzumab emtansine Trastuzumab emtansine
|
 |
 |
? mg/ml |
2-8°C |
 |
| 24 |
 |
|
2990 |
 Trastuzumab emtansine Trastuzumab emtansine
|
 |
 |
? mg/ml |
2-8°C |
 |
| 24 |
 |
|
2990 |
 Trastuzumab emtansine Trastuzumab emtansine
|
 |
 |
? mg/ml |
2-8°C |
 |
| 24 |
 |
|
2990 |
 Treosulfan Treosulfan
|
 |
 |
50 mg/ml |
25°C |
 |
| 46 |
 |
|
1990 |
 Treosulfan Treosulfan
|
 |
 |
50 mg/ml |
25°C |
 |
| 46 |
 |
|
1990 |
 Treosulfan Treosulfan
|
 |
 |
50 mg/ml |
25°C |
 |
| 46 |
 |
|
1990 |
 Treprostinil Treprostinil
|
 |
 |
0,004 & 0,13 mg/ml |
40°C |
 |
| 48 |
 |
|
1904 |
 Treprostinil Treprostinil
|
 |
 |
0,13 mg/ml |
40°C |
 |
| 48 |
 |
|
1904 |
 Treprostinil Treprostinil
|
 |
 |
0,004 mg/ml |
40°C |
 |
| 48 |
 |
|
1904 |
 Treprostinil Treprostinil
|
 |
 |
0,02 mg/ml |
40°C |
 |
| 48 |
 |
|
1904 |
 Trimethoprim Trimethoprim
|
 |
 |
mg |
25°C |
 |
| 42 |
 |
|
3002 |
 Trimethoprim Trimethoprim
|
 |
 |
mg |
25°C |
 |
| 42 |
 |
|
3002 |
 Urokinase Urokinase
|
 |
 |
0,005 MUI/ml |
-20°C |
 |
| 56 |
 |
|
2050 |
 Urokinase Urokinase
|
 |
 |
0,005 MUI/ml |
37°C |
 |
| 56 |
 |
|
2050 |
 Urokinase Urokinase
|
 |
 |
0,005 MUI/ml |
7°C |
 |
| 56 |
 |
|
2050 |
 Ursodesoxycholique acid Ursodesoxycholique acid
|
 |
 |
mg |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
5 mg/ml |
24°C |
 |
| 17 |
 |
|
430 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
3120 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
10 mg/ml |
4°C |
 |
| 8 |
 |
|
3120 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
25 mg/ml |
-20°C |
 |
| 90 |
 |
|
1871 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
5 mg/ml |
24°C |
 |
| 17 |
 |
|
430 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
4 & 5 mg/ml |
23°C |
 |
| 24 |
 |
|
694 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
20 & 40 mg/ml |
25°C |
 |
| 96 |
 |
|
654 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
20 & 40 mg/ml |
5°C |
 |
| 30 |
 |
|
654 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
4 & 5 mg/ml |
23°C |
 |
| 17 |
 |
|
694 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
0,025 mg/ml |
4°C |
 |
| 28 |
 |
|
2370 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
0,025 mg/ml |
4°C |
 |
| 28 |
 |
|
2370 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
5 mg/ml |
25°C |
 |
| 48 |
 |
|
2173 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
5 mg/ml |
4°C |
 |
| 180 |
 |
|
2173 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
10 & 20 mg/ml |
25°C |
 |
| 24 |
 |
|
604 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
mg/ml |
- 20 °C |
 |
| 14 |
 |
|
4681 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
mg/ml |
- 20 °C |
 |
| 14 |
 |
|
4681 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
mg/ml |
-20°C |
 |
| 90 |
 |
|
4596 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
mg/ml |
2-8 °C |
 |
| 28 |
 |
|
4681 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
mg/ml |
2-8 °C |
 |
| 28 |
 |
|
4681 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4177 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
mg/ml |
23°C |
 |
| 26 |
 |
|
3153 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
mg/ml |
5°C |
 |
| 30 |
 |
|
4596 |
 Vecuronium bromide Vecuronium bromide
|
 |
 |
1 mg/ml |
23-25°C |
 |
| 21 |
 |
|
2209 |
 Vecuronium bromide Vecuronium bromide
|
 |
 |
1 mg/ml |
3-5°C |
 |
| 21 |
 |
|
2209 |
 Vedolizumab Vedolizumab
|
 |
 |
1.2 mg/ml |
2-8°C |
 |
| 24 |
 |
|
3711 |
 Vedolizumab Vedolizumab
|
 |
 |
1.2 mg/ml |
20-25°C |
 |
| 12 |
 |
|
3711 |
 Verapamil hydrochloride Verapamil hydrochloride
|
 |
 |
mg/ml |
2-8 °C |
 |
| 60 |
 |
|
3209 |
 Verapamil hydrochloride Verapamil hydrochloride
|
 |
 |
mg/ml |
2-8°C |
 |
| 60 |
 |
|
3209 |
 Vincristine sulfate liposome Vincristine sulfate liposome
|
 |
 |
0,16 mg/ml |
15-30°C |
 |
| 12 |
 |
|
3775 |
 Vincristine sulfate liposome Vincristine sulfate liposome
|
 |
 |
mg/ml |
15-30°C |
 |
| 12 |
 |
|
3775 |
 Vinorelbine tartrate Vinorelbine tartrate
|
 |
 |
0,385 mg/ml |
21°C |
 |
| 7 |
 |
|
1520 |
 Voriconazole Voriconazole
|
 |
 |
mg/ml |
4°C |
 |
| 28 |
 |
|
4799 |
 Voriconazole Voriconazole
|
 |
 |
4 mg/ml |
4°C |
 |
| 15 |
 |
|
2111 |
 Voriconazole Voriconazole
|
 |
 |
mg/ml |
4°C |
 |
| 28 |
 |
|
4799 |
 Voriconazole Voriconazole
|
 |
 |
mg/ml |
2-8°C |
 |
| 90 |
 |
|
4705 |
 Voriconazole Voriconazole
|
 |
 |
0,5 mg/ml |
2-8°C |
 |
| 13 |
 |
|
3975 |
 Zidovudine Zidovudine
|
 |
 |
4 mg/ml |
25°C |
 |
| 8 |
 |
|
338 |
|