A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - 
|
 |
 |
 |
 |
 |
 |
 |
 |
 Adalimumab Adalimumab
|
 |
 |
50 mg/ml |
23-27°C |
 |
| 28 |
 |
|
4627 |
 Aflibercept Aflibercept
|
 |
 |
0,6 >> 8 mg/ml |
25°C |
 |
| 8 |
 |
|
3508 |
 Aflibercept Aflibercept
|
 |
 |
0,6 >> 8 mg/ml |
25°C |
 |
| 8 |
 |
|
3508 |
 Alizapride hydrochloride Alizapride hydrochloride
|
 |
 |
0,5 & 2 mg/ml |
25°C |
 |
| 4 |
 |
|
1688 |
 Alprostadil Alprostadil
|
 |
 |
1.5 µg/ml |
28-32°C |
 |
| 48 |
 |
|
4069 |
 Alprostadil Alprostadil
|
 |
 |
15 µg/ml |
28-32°C |
 |
| 48 |
 |
|
4069 |
 Alprostadil Alprostadil
|
 |
 |
1,5 µg/ml |
28-32°C |
 |
| 48 |
 |
|
3761 |
 Alprostadil Alprostadil
|
 |
 |
15 µg/ml |
28-32°C |
 |
| 48 |
 |
|
3761 |
 Amikacin sulfate Amikacin sulfate
|
 |
 |
5 mg/ml |
25°C |
 |
| 48 |
 |
|
434 |
 Amikacin sulfate Amikacin sulfate
|
 |
 |
190 mg/ml |
22°C-23°C |
 |
| 48 |
 |
|
639 |
 Aminophylline Aminophylline
|
 |
 |
1,3 mg/ml |
25°C |
 |
| 48 |
 |
|
434 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
0,84 mg/ml |
25°C |
 |
| 6 |
 |
|
1733 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
1,8 mg/ml |
24°C |
 |
| 24 |
 |
|
242 |
 Amiodarone hydrochloride Amiodarone hydrochloride
|
 |
 |
0,9 mg/ml |
25°C |
 |
| 24 |
 |
|
2146 |
 Amitriptyline hydrochloride Amitriptyline hydrochloride
|
 |
 |
? mg/ml |
25°C |
 |
| 4 |
 |
|
54 |
 Amitriptyline hydrochloride Amitriptyline hydrochloride
|
 |
 |
? mg/ml |
25°C |
 |
| 4 |
 |
|
54 |
 Amlodipine besylate Amlodipine besylate
|
 |
 |
mg/ml |
25°C |
 |
| 56 |
 |
|
2689 |
 Amlodipine besylate Amlodipine besylate
|
 |
 |
mg/ml |
25°C |
 |
| 56 |
 |
|
2689 |
 Amlodipine besylate Amlodipine besylate
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
2689 |
 Amlodipine besylate Amlodipine besylate
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
2689 |
 Amoxicillin sodium Amoxicillin sodium
|
 |
 |
20 mg/ml |
20-25°C |
 |
| 12 |
 |
|
4634 |
 Amoxicillin sodium / clavulanic acid Amoxicillin sodium / clavulanic acid
|
 |
 |
20 / 2 mg/ml |
20-25°C |
 |
| 4 |
 |
|
1746 |
 Amoxicillin sodium / clavulanic acid Amoxicillin sodium / clavulanic acid
|
 |
 |
20 / 4 mg/ml |
20-25°C |
 |
| 4 |
 |
|
1746 |
 Amphotericin B Amphotericin B
|
 |
 |
0,2 & 0,5 mg/ml |
25°C |
 |
| 120 |
 |
|
283 |
 Amphotericin B Amphotericin B
|
 |
 |
0,47 >> 0,75 mg/ml |
25°C |
 |
| 24 |
 |
|
497 |
 Amphotericin B Amphotericin B
|
 |
 |
mg/ml |
20-25°C |
 |
| 13 |
 |
|
2867 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
24 mg/ml |
20-24°C |
 |
| 24 |
 |
|
4640 |
 Ampicillin sodium Ampicillin sodium
|
 |
 |
24 mg/ml |
3-4°C |
 |
| 72 |
 |
|
4640 |
 Angiotensin II Angiotensin II
|
 |
 |
10 µg/mL |
2-8°C |
 |
| 5 |
 |
|
4808 |
 Argatroban Argatroban
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 180 |
 |
|
3235 |
 Argatroban Argatroban
|
 |
 |
0,5 mg/ml |
25°C |
 |
| 180 |
 |
|
3235 |
 Argatroban Argatroban
|
 |
 |
1 mg/ml |
15-30°C |
 |
| 24 |
 |
|
2277 |
 Argatroban Argatroban
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 14 |
 |
|
3295 |
 Argatroban Argatroban
|
 |
 |
1 mg/ml |
25°C |
 |
| 14 |
 |
|
3295 |
 Argatroban Argatroban
|
 |
 |
1 mg/ml |
15-30°C |
 |
| 24 |
 |
|
2277 |
 Atezolizumab Atezolizumab
|
 |
 |
2,4 >> 16,8 mg/ml |
30°C |
 |
| 24 |
 |
|
4583 |
 Atezolizumab Atezolizumab
|
 |
 |
2,4 >> 16,8 mg/ml |
30°C |
 |
| 24 |
 |
|
4583 |
 Atropine sulfate Atropine sulfate
|
 |
 |
2 mg/ml |
23°C |
 |
| 364 |
 |
|
2297 |
 Atropine sulfate Atropine sulfate
|
 |
 |
2 mg/ml |
35°C |
 |
| 28 |
 |
|
2297 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 72 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 24 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 72 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 24 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 72 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 24 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 72 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 24 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 72 |
 |
|
4474 |
 Avelumab Avelumab
|
 |
 |
3.2 mg/mL |
20-25°C |
 |
| 24 |
 |
|
4474 |
 Azathioprine sodium Azathioprine sodium
|
 |
 |
2 mg/ml |
23°C |
 |
| 8 |
 |
|
1260 |
 Azathioprine sodium Azathioprine sodium
|
 |
 |
2 mg/ml |
23°C |
 |
| 8 |
 |
|
1260 |
 Azathioprine sodium Azathioprine sodium
|
 |
 |
2 mg/ml |
23°C |
 |
| 8 |
 |
|
1260 |
 Aztreonam Aztreonam
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
432 |
 Aztreonam Aztreonam
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 48 |
 |
|
4634 |
 Aztreonam Aztreonam
|
 |
 |
120 mg/ml |
25°C |
 |
| 12 |
 |
|
4326 |
 Aztreonam Aztreonam
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
644 |
 Aztreonam Aztreonam
|
 |
 |
40 mg/ml |
25°C |
 |
| 12 |
 |
|
4326 |
 Bendamustine hydrochloride Bendamustine hydrochloride
|
 |
 |
≈ 0.3 >> 0.4 mg/ml |
15-30°C |
 |
| 3 |
 |
|
2288 |
 Bevacizumab Bevacizumab
|
 |
 |
1,4 >>16,5 mg/ml |
30°C |
 |
| 2 |
 |
|
4516 |
 Bevacizumab Bevacizumab
|
 |
 |
1,4 >>16,5 mg/ml |
30°C |
 |
| 2 |
 |
|
4516 |
 Bortezomib Bortezomib
|
 |
 |
1 mg/ml |
23°C |
 |
| 42 |
 |
|
2232 |
 Bortezomib Bortezomib
|
 |
 |
1 & 2.5 mg/ml |
25°C |
 |
| 8 |
 |
|
1911 |
 Bortezomib Bortezomib
|
 |
 |
2,5 mg/ml |
23°C |
 |
| 21 |
 |
|
3688 |
 Bortezomib Bortezomib
|
 |
 |
1 mg/ml |
22°C |
 |
| 3 |
 |
|
2010 |
 Bortezomib Bortezomib
|
 |
 |
2,5 mg/ml |
23°C |
 |
| 21 |
 |
|
3688 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
743 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
1 mg/ml |
25°C |
 |
| 30 |
 |
|
1193 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
1193 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
1 mg/ml |
25°C |
 |
| 30 |
 |
|
1193 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
743 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
1 mg/ml |
25°C |
 |
| 30 |
 |
|
1193 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
743 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
1 mg/ml |
25°C |
 |
| 30 |
 |
|
1193 |
 Bretylium tosilate Bretylium tosilate
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
743 |
 Bumetanide Bumetanide
|
 |
 |
0,02 & 2 mg/ml |
23°C-25°C |
 |
| 72 |
 |
|
42 |
 Bupivacaine hydrochloride Bupivacaine hydrochloride
|
 |
 |
1,25 mg/ml |
23°C |
 |
| 32 |
 |
|
293 |
 Busulfan Busulfan
|
 |
 |
6 mg/ml |
18°C-20°C |
 |
| 28 |
 |
|
2128 |
 Busulfan Busulfan
|
 |
 |
6 mg/ml |
2-8°C |
 |
| 28 |
 |
|
2128 |
 Caffeine Caffeine
|
 |
 |
5 mg/ml |
21°C |
 |
| 24 |
 |
|
698 |
 Caffeine Caffeine
|
 |
 |
5 mg/ml |
21°C |
 |
| 24 |
 |
|
698 |
 Caffeine citrate Caffeine citrate
|
 |
 |
5 mg/ml |
27°C |
 |
| 24 |
 |
|
691 |
 Caffeine citrate Caffeine citrate
|
 |
 |
5 mg/ml |
27°C |
 |
| 24 |
 |
|
691 |
 Caffeine citrate Caffeine citrate
|
 |
 |
10 mg/ml |
22°C |
 |
| 180 |
 |
|
43 |
 Calcitriol Calcitriol
|
 |
 |
0,5 µg/ml |
25°C |
 |
| 8 |
 |
|
305 |
 Calcitriol Calcitriol
|
 |
 |
0,5 µg/ml |
25°C |
 |
| 8 |
 |
|
305 |
 Calcitriol Calcitriol
|
 |
 |
1 & 2 µg/ml |
25°C |
 |
| 8 |
 |
|
305 |
 Calcium chloride Calcium chloride
|
 |
 |
11,5 mg/ml |
25°C |
 |
| 7 |
 |
|
3307 |
 Calcium chloride Calcium chloride
|
 |
 |
9,3 mg/ml |
25°C |
 |
| 7 |
 |
|
3307 |
 Calcium chloride Calcium chloride
|
 |
 |
8,9 mg/ml |
25°C |
 |
| 7 |
 |
|
3307 |
 Carboplatin Carboplatin
|
 |
 |
0,25 mg/ml |
2-8°C |
 |
| 3 |
 |
|
3830 |
 Carboplatin Carboplatin
|
 |
 |
0,25 mg/ml |
25°C |
 |
| 24 |
 |
|
3830 |
 Carboplatin Carboplatin
|
 |
 |
2 mg/ml |
2-8°C |
 |
| 7 |
 |
|
3830 |
 Carboplatin Carboplatin
|
 |
 |
2 mg/ml |
25°C |
 |
| 24 |
 |
|
3830 |
 Carboplatin Carboplatin
|
 |
 |
4 mg/ml |
2-8°C |
 |
| 7 |
 |
|
3830 |
 Carboplatin Carboplatin
|
 |
 |
4 mg/ml |
25°C |
 |
| 2 |
 |
|
3830 |
 Carmustine Carmustine
|
 |
 |
1 mg/ml |
23°C |
 |
| 6 |
 |
|
2146 |
 Cefalotin sodium Cefalotin sodium
|
 |
 |
10 mg/ml |
20°C-22°C |
 |
| 24 |
 |
|
1670 |
 Cefalotin sodium Cefalotin sodium
|
 |
 |
10 mg/ml |
4°C |
 |
| 48 |
 |
|
1670 |
 Cefamandole nafate Cefamandole nafate
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
418 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
418 |
 Cefazolin sodium Cefazolin sodium
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4634 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
2,5/5/10/20 mg/ml |
4°C |
 |
| 7 |
 |
|
1605 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
20 mg/ml |
22°C-24°C |
 |
| 3 |
 |
|
1605 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
10 mg/ml |
22°C-24°C |
 |
| 1 |
 |
|
1605 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
2,5 mg/ml |
22°C-24°C |
 |
| 6 |
 |
|
1605 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
5 mg/ml |
22°C-24°C |
 |
| 3 |
 |
|
1605 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
10 mg/ml |
22°C-24°C |
 |
| 2 |
 |
|
1605 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
2,5 mg/ml |
22°C-24°C |
 |
| 4 |
 |
|
1605 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
5 mg/ml |
22°C-24°C |
 |
| 1 |
 |
|
1605 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
8 mg/ml |
24°C |
 |
| 48 |
 |
|
1673 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4634 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
120 mg/ml |
25°C |
 |
| 10 |
 |
|
4326 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
20 mg/ml |
25°C |
 |
| 12 |
 |
|
4326 |
 Cefepime dihydrochloride Cefepime dihydrochloride
|
 |
 |
40 mg/ml |
25°C |
 |
| 10 |
 |
|
4326 |
 Cefiderocol sulfate tosylate Cefiderocol sulfate tosylate
|
 |
 |
62,5 mg/mL |
20-25°C |
 |
| 24 |
 |
|
4634 |
 Cefonicid sodium Cefonicid sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
644 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
100 >>300 mg/ml |
15-25°C |
 |
| 24 |
 |
|
3544 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
644 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
2 >> 50 mg/ml |
15-25°C |
 |
| 24 |
 |
|
3544 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
2 >> 50 mg/ml |
15-25°C |
 |
| 24 |
 |
|
3544 |
 Cefoperazone sodium Cefoperazone sodium
|
 |
 |
2 >> 50 mg/ml |
15-25°C |
 |
| 24 |
 |
|
3544 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
20 mg/ml |
24°C |
 |
| 24 |
 |
|
421 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
20 mg/ml |
24°C |
 |
| 24 |
 |
|
421 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
10 mg/ml |
25°C |
 |
| 24 |
 |
|
592 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 6 |
 |
|
4268 |
 Cefotaxime sodium Cefotaxime sodium
|
 |
 |
83,3 mg/ml |
20-25°C |
 |
| 6 |
 |
|
4268 |
 Cefoxitin sodium Cefoxitin sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
418 |
 Cefoxitin sodium Cefoxitin sodium
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 12 |
 |
|
4634 |
 Ceftazidime Ceftazidime
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
432 |
 Ceftazidime Ceftazidime
|
 |
 |
4 mg/ml |
25°C |
 |
| 24 |
 |
|
1656 |
 Ceftazidime Ceftazidime
|
 |
 |
4 mg/ml |
4°C |
 |
| 7 |
 |
|
1656 |
 Ceftazidime Ceftazidime
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 24 |
 |
|
2450 |
 Ceftazidime Ceftazidime
|
 |
 |
0,1 mg/ml |
37°C |
 |
| 2 |
 |
|
2450 |
 Ceftazidime Ceftazidime
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4634 |
 Ceftazidime Ceftazidime
|
 |
 |
20 mg/ml |
-20°C |
 |
| 90 |
 |
|
4032 |
 Ceftazidime Ceftazidime
|
 |
 |
20 mg/ml |
2-8°C |
 |
| 7 |
 |
|
4032 |
 Ceftazidime Ceftazidime
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 8 |
 |
|
4634 |
 Ceftazidime Avibactam Ceftazidime Avibactam
|
 |
 |
125/31,25 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4634 |
 Ceftizoxime sodium Ceftizoxime sodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
418 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftobiprole medocaril sodium Ceftobiprole medocaril sodium
|
 |
 |
2,67 mg/ml |
25°C |
 |
| 8 |
 |
|
4650 |
 Ceftolozane / tazobactam Ceftolozane / tazobactam
|
 |
 |
62,5/31,25 mg/ml |
20-25°C |
 |
| 48 |
 |
|
4634 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 24 |
 |
|
432 |
 Ceftriaxone disodium Ceftriaxone disodium
|
 |
 |
20 mg/ml |
25°C |
 |
| 48 |
 |
|
432 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
15 mg/ml |
25°C |
 |
| 48 |
 |
|
644 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
6 mg/ml |
25°C |
 |
| 24 |
 |
|
1656 |
 Cefuroxime sodium Cefuroxime sodium
|
 |
 |
6 mg/ml |
4°C |
 |
| 7 |
 |
|
1656 |
 Cetuximab Cetuximab
|
 |
 |
5 mg/ml |
-20°C |
 |
| 72 |
 |
|
2220 |
 Cetuximab Cetuximab
|
 |
 |
5 mg/ml |
2-8°C |
 |
| 1461 |
 |
|
2220 |
 Cetuximab Cetuximab
|
 |
 |
5 mg/ml |
25°C |
 |
| 72 |
 |
|
2220 |
 Cetuximab Cetuximab
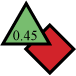
|
 |
 |
0.45 >> 5 mg/ml |
25°C |
 |
| 48 |
 |
|
2220 |
 Cetuximab Cetuximab
|
 |
 |
0.45 >> 5 mg/ml |
25°C |
 |
| 48 |
 |
|
2220 |
 Cetuximab Cetuximab
|
 |
 |
0.45 >> 5 mg/ml |
25°C |
 |
| 48 |
 |
|
2220 |
 Cetuximab Cetuximab
|
 |
 |
0.45 >> 5 mg/ml |
25°C |
 |
| 48 |
 |
|
2220 |
 Ciclosporin Ciclosporin
|
 |
 |
2 mg/ml |
24°C |
 |
| 48 |
 |
|
471 |
 Cidofovir Cidofovir
|
 |
 |
0,21 & 8,12 mg/ml |
30°C |
 |
| 24 |
 |
|
1 |
 Cidofovir Cidofovir
|
 |
 |
0,21 & 8,12 mg/ml |
30°C |
 |
| 24 |
 |
|
1 |
 Cimetidine hydrochloride Cimetidine hydrochloride
|
 |
 |
1,2 & 5 mg/ml |
25°C |
 |
| 7 |
 |
|
747 |
 Cimetidine hydrochloride Cimetidine hydrochloride
|
 |
 |
1,2 & 5 mg/ml |
25°C |
 |
| 7 |
 |
|
747 |
 Cimetidine hydrochloride Cimetidine hydrochloride
|
 |
 |
1,2 & 5 mg/ml |
25°C |
 |
| 7 |
 |
|
747 |
 Cimetidine hydrochloride Cimetidine hydrochloride
|
 |
 |
1,2 & 5 mg/ml |
25°C |
 |
| 7 |
 |
|
747 |
 Cimetidine hydrochloride Cimetidine hydrochloride
|
 |
 |
1,2 & 5 mg/ml |
25°C |
 |
| 7 |
 |
|
747 |
 Cimetidine hydrochloride Cimetidine hydrochloride
|
 |
 |
1,2 & 5 mg/ml |
25°C |
 |
| 7 |
 |
|
747 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
1 mg/ml |
21-24°C |
 |
| 30 |
 |
|
3231 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
1,5 mg/ml |
25°C |
 |
| 48 |
 |
|
434 |
 Ciprofloxacin lactate Ciprofloxacin lactate
|
 |
 |
2 mg/ml |
21-24°C |
 |
| 30 |
 |
|
3231 |
 Citalopram Citalopram
|
 |
 |
0,16 mg/ml |
20°C-25°C |
 |
| 24 |
 |
|
1604 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9 mg/ml |
24°C |
 |
| 24 |
 |
|
421 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9 mg/ml |
25°C |
 |
| 48 |
 |
|
418 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9 & 12 mg/ml |
25°C |
 |
| 48 |
 |
|
432 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
9 mg/ml |
24°C |
 |
| 24 |
 |
|
421 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
150 mg/ml |
22°C-23°C |
 |
| 48 |
 |
|
639 |
 Clindamycin phosphate Clindamycin phosphate
|
 |
 |
60 mg/ml |
25°C |
 |
| 48 |
 |
|
644 |
 Clofarabine Clofarabine
|
 |
 |
0,2 & 0,6 mg/ml |
25°C |
 |
| 28 |
 |
|
3110 |
 Clonazepam Clonazepam
|
 |
 |
0,5 mg/ml |
25°C |
 |
| 48 |
 |
|
2217 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
250 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4634 |
 Cloxacillin sodium Cloxacillin sodium
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4634 |
 Clozapine Clozapine
|
 |
 |
mg/ml |
23°C |
 |
| 63 |
 |
|
2592 |
 Clozapine Clozapine
|
 |
 |
mg/ml |
23°C |
 |
| 63 |
 |
|
2592 |
 Clozapine Clozapine
|
 |
 |
mg/ml |
23°C |
 |
| 63 |
 |
|
2592 |
 Clozapine Clozapine
|
 |
 |
mg/ml |
23°C |
 |
| 63 |
 |
|
2592 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
3.33/0.66 mg/ml |
20-22°C |
 |
| 4 |
 |
|
4012 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
4,21/0,84 mg/ml |
20-22°C |
 |
| 4 |
 |
|
4012 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
5,71/1,14 mg/ml |
20-22°C |
 |
| 2 |
 |
|
4012 |
 Co-trimoxazole Co-trimoxazole
|
 |
 |
16/80 mg/ml |
18°C-24°C |
 |
| 60 |
 |
|
446 |
 Cytarabine Cytarabine
|
 |
 |
5 mg/ml |
25°C |
 |
| 7 |
 |
|
739 |
 Cytarabine Cytarabine
|
 |
 |
5 mg/ml |
25°C |
 |
| 7 |
 |
|
739 |
 Cytarabine Cytarabine
|
 |
 |
5 mg/ml |
25°C |
 |
| 7 |
 |
|
739 |
 Dalteparin sodium Dalteparin sodium
|
 |
 |
10000 UI/ml |
25°C |
 |
| 10 |
 |
|
1624 |
 Dalteparin sodium Dalteparin sodium
|
 |
 |
1000 UI/ml |
2-8°C |
 |
| 30 |
 |
|
4031 |
 Daratumumab Daratumumab
|
 |
 |
0,8 >> 3,2 mg/mL |
15-25°C |
 |
| 15 |
 |
|
4190 |
 Daratumumab Daratumumab
|
 |
 |
0,8 >> 3,2 mg/mL |
15-25°C |
 |
| 15 |
 |
|
4190 |
 Daratumumab Daratumumab
|
 |
 |
0,8 >> 3,2 mg/mL |
15-25°C |
 |
| 15 |
 |
|
4190 |
 Daratumumab Daratumumab
|
 |
 |
0,8 >> 3,2 mg/mL |
15-25°C |
 |
| 15 |
 |
|
4190 |
 Daunorubicin hydrochloride Daunorubicin hydrochloride
|
 |
 |
0,02 mg/ml |
22°C |
 |
| 48 |
 |
|
1897 |
 Daunorubicin hydrochloride Daunorubicin hydrochloride
|
 |
 |
0,02 mg/ml |
22°C |
 |
| 48 |
 |
|
1897 |
 Daunorubicin hydrochloride Daunorubicin hydrochloride
|
 |
 |
0,1 mg/ml |
22°C |
 |
| 8 |
 |
|
1897 |
 Deferoxamine mesylate Deferoxamine mesylate
|
 |
 |
210 mg/ml |
20°C-23°C |
 |
| 21 |
 |
|
1669 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
10 mg/ml |
23°C |
 |
| 91 |
 |
|
28 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
10 mg/ml |
4°C |
 |
| 91 |
 |
|
28 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
10 mg/ml |
23°C |
 |
| 55 |
 |
|
28 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
10 mg/ml |
4°C |
 |
| 55 |
 |
|
28 |
 Dexamethasone sodium phosphate Dexamethasone sodium phosphate
|
 |
 |
1 >> 4 mg/ml |
22°C |
 |
| 28 |
 |
|
546 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 8 |
 |
|
3611 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
3611 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
3 mg/ml |
25°C |
 |
| 8 |
 |
|
3611 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
3611 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
3 mg/ml |
25°C |
 |
| 8 |
 |
|
3611 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
4 & 8 mg/ml |
25°C |
 |
| 8 |
 |
|
2201 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
4 mg/ml |
25°C |
 |
| 4 |
 |
|
2201 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
8 mg/ml |
25°C |
 |
| 8 |
 |
|
2201 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
0,1 & 0,5 mg/ml |
25°C |
 |
| 24 |
 |
|
1949 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 12 |
 |
|
1949 |
 Dexrazoxane hydrochloride Dexrazoxane hydrochloride
|
 |
 |
3 >> 10 mg/ml |
25°C |
 |
| 4 |
 |
|
3581 |
 Diamorphine hydrochloride Diamorphine hydrochloride
|
 |
 |
2 & 20 mg/ml |
18-22°C |
 |
| 18 |
 |
|
1886 |
 Diazepam Diazepam
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 24 |
 |
|
621 |
 Diazepam Diazepam
|
 |
 |
0,1 mg/ml |
21°C |
 |
| 24 |
 |
|
1472 |
 Diazepam Diazepam
|
 |
 |
0,04 mg/ml |
20-25°C |
 |
| 48 |
 |
|
1960 |
 Diazepam Diazepam
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 24 |
 |
|
621 |
 Diazepam Diazepam
|
 |
 |
0,1 mg/ml |
21°C |
 |
| 24 |
 |
|
1472 |
 Diazepam Diazepam
|
 |
 |
0,04 mg/ml |
25°C |
 |
| 48 |
 |
|
1522 |
 Digoxin Digoxin
|
 |
 |
2,5 µg/ml |
23-26°C |
 |
| 48 |
 |
|
618 |
 Digoxin Digoxin
|
 |
 |
50 µg/ml |
23-27°C |
 |
| 180 |
 |
|
4690 |
 Digoxin Digoxin
|
 |
 |
50 µg/ml |
3-7°C |
 |
| 180 |
 |
|
4690 |
 Digoxin Digoxin
|
 |
 |
2,5 µg/ml |
23°C-26°C |
 |
| 6 |
 |
|
618 |
 Diltiazem hydrochloride Diltiazem hydrochloride
|
 |
 |
1 mg/ml |
21°C |
 |
| 48 |
 |
|
1462 |
 Diltiazem hydrochloride Diltiazem hydrochloride
|
 |
 |
1 mg/ml |
21°C |
 |
| 48 |
 |
|
1462 |
 Diltiazem hydrochloride Diltiazem hydrochloride
|
 |
 |
1 mg/ml |
22-25°C |
 |
| 30 |
 |
|
3409 |
 Dimenhydrinate Dimenhydrinate
|
 |
 |
0,5 >> 2 mg/ml |
22°C |
 |
| 7 |
 |
|
1806 |
 Dimenhydrinate Dimenhydrinate
|
 |
 |
2,5 >> 10 mg/ml |
22°C |
 |
| 60 |
 |
|
1806 |
 Disopyramide phosphate Disopyramide phosphate
|
 |
 |
mg |
25°C |
 |
| 28 |
 |
|
2471 |
 Disopyramide phosphate Disopyramide phosphate
|
 |
 |
mg |
25°C |
 |
| 28 |
 |
|
2471 |
 Disulfiram Disulfiram
|
 |
 |
mg/ml |
23-25°C |
 |
| 295 |
 |
|
2469 |
 Disulfiram Disulfiram
|
 |
 |
mg/ml |
23-25°C |
 |
| 178 |
 |
|
2469 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
1058 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
1058 |
 Dobutamine hydrochloride Dobutamine hydrochloride
|
 |
 |
5 mg/ml |
25°C |
 |
| 24 |
 |
|
196 |
 Docetaxel Docetaxel
|
 |
 |
10 mg/ml |
23°C |
 |
| 21 |
 |
|
2210 |
 Docetaxel Docetaxel
|
 |
 |
0.4 & 0.8 mg/ml |
23°C |
 |
| 35 |
 |
|
2210 |
 Domperidone Domperidone
|
 |
 |
mg/ml |
25°C |
 |
| 75 |
 |
|
4142 |
 Domperidone Domperidone
|
 |
 |
mg/ml |
25°C |
 |
| 75 |
 |
|
4142 |
 Domperidone Domperidone
|
 |
 |
mg/ml |
25°C |
 |
| 75 |
 |
|
4142 |
 Domperidone Domperidone
|
 |
 |
mg/ml |
25°C |
 |
| 75 |
 |
|
4142 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
0,8 mg/ml |
23-25°C |
 |
| 48 |
 |
|
732 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
1 mg/ml |
25°C |
 |
| 36 |
 |
|
1249 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
0,8 mg/ml |
23-25°C |
 |
| 48 |
 |
|
732 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
0,8 mg/ml |
25°C |
 |
| 24 |
 |
|
732 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
6,4 mg/ml |
23°C |
 |
| 24 |
 |
|
192 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
4 mg/ml |
25°C |
 |
| 24 |
 |
|
196 |
 Dopamine hydrochloride Dopamine hydrochloride
|
 |
 |
0,4 >> 1 mg/ml |
25°C |
 |
| 24 |
 |
|
1698 |
 Doripenem Doripenem
|
 |
 |
5 mg/ml |
25°C |
 |
| 12 |
 |
|
2316 |
 Doripenem Doripenem
|
 |
 |
5 mg/ml |
25°C |
 |
| 4 |
 |
|
2316 |
 Doripenem Doripenem
|
 |
 |
5 mg/ml |
25°C |
 |
| 12 |
 |
|
2316 |
 Doripenem Doripenem
|
 |
 |
5 mg/ml |
25°C |
 |
| 4 |
 |
|
2316 |
 Doxapram hydrochloride Doxapram hydrochloride
|
 |
 |
2.5 mg/ml |
25°C |
 |
| 36 |
 |
|
3723 |
 Doxapram hydrochloride Doxapram hydrochloride
|
 |
 |
8 mg/ml |
25°C |
 |
| 24 |
 |
|
3723 |
 Doxorubicin hydrochloride Doxorubicin hydrochloride
|
 |
 |
2 mg/ml |
23°C |
 |
| 124 |
 |
|
681 |
 Doxorubicin hydrochloride Doxorubicin hydrochloride
|
 |
 |
2 mg/ml |
4°C |
 |
| 124 |
 |
|
681 |
 Doxorubicin hydrochloride Doxorubicin hydrochloride
|
 |
 |
0,02 mg/ml |
22°C |
 |
| 24 |
 |
|
1897 |
 Doxorubicin hydrochloride Doxorubicin hydrochloride
|
 |
 |
1 & 2 mg/ml |
23°C |
 |
| 124 |
 |
|
681 |
 Droperidol Droperidol
|
 |
 |
0,02 mg/ml |
27°C |
 |
| 10 |
 |
|
1187 |
 Droperidol Droperidol
|
 |
 |
0,02 mg/ml |
27°C |
 |
| 7 |
 |
|
1187 |
 Droperidol Droperidol
|
 |
 |
0,02 mg/ml |
27°C |
 |
| 7 |
 |
|
1187 |
 Droperidol Droperidol
|
 |
 |
0,18 mg/ml |
20°C-25°C |
 |
| 14 |
 |
|
1968 |
 Durvalumab Durvalumab
|
 |
 |
50 mg/ml |
20-25°C |
 |
| 14 |
 |
|
4772 |
 Enoxaparin sodium Enoxaparin sodium
|
 |
 |
1,2 mg/ml |
20°C-22°C |
 |
| 48 |
 |
|
311 |
 Enoxaparin sodium Enoxaparin sodium
|
 |
 |
100 mg/ml |
3°C |
 |
| 10 |
 |
|
811 |
 Ephedrine sulfate Ephedrine sulfate
|
 |
 |
5 mg/ml |
25°C |
 |
| 60 |
 |
|
1720 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
16 µg/ml |
23-25°C |
 |
| 45 |
 |
|
4026 |
 Epinephrine hydrochloride Epinephrine hydrochloride
|
 |
 |
64 µg/ml |
23-25°C |
 |
| 45 |
 |
|
4026 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
0,02 mg/ml |
22°C |
 |
| 96 |
 |
|
1897 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
0,02 mg/ml |
22°C |
 |
| 24 |
 |
|
1897 |
 Epirubicin hydrochloride Epirubicin hydrochloride
|
 |
 |
0,02 mg/ml |
22°C |
 |
| 96 |
 |
|
1897 |
 Ertapenem Ertapenem
|
 |
 |
100 mg/ml |
25°C |
 |
| 0.5 |
 |
|
3717 |
 Esmolol hydrochloride Esmolol hydrochloride
|
 |
 |
10 mg/ml |
23°C-27°C |
 |
| 7 |
 |
|
204 |
 Esmolol hydrochloride Esmolol hydrochloride
|
 |
 |
10 mg/ml |
23°C-27°C |
 |
| 7 |
 |
|
204 |
 Esmolol hydrochloride Esmolol hydrochloride
|
 |
 |
10 mg/ml |
23°C-27°C |
 |
| 7 |
 |
|
204 |
 Esmolol hydrochloride Esmolol hydrochloride
|
 |
 |
10 mg/ml |
23°C-27°C |
 |
| 7 |
 |
|
204 |
 Esmolol hydrochloride Esmolol hydrochloride
|
 |
 |
10 mg/ml |
23°C-27°C |
 |
| 7 |
 |
|
204 |
 Esmolol hydrochloride Esmolol hydrochloride
|
 |
 |
10 mg/ml |
23°C-27°C |
 |
| 7 |
 |
|
204 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
20°C-22°C |
 |
| 4 |
 |
|
482 |
 Etoposide Etoposide
|
 |
 |
0,2 mg/ml |
24°C |
 |
| 22 |
 |
|
1613 |
 Etoposide Etoposide
|
 |
 |
0,3 mg/ml |
24°C |
 |
| 2 |
 |
|
1613 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
24°C |
 |
| 24 |
 |
|
1613 |
 Etoposide Etoposide
|
 |
 |
10 mg/ml |
24°C |
 |
| 5 |
 |
|
1613 |
 Etoposide Etoposide
|
 |
 |
12 mg/ml |
24°C |
 |
| 7 |
 |
|
1613 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
20°C-22°C |
 |
| 4 |
 |
|
482 |
 Etoposide Etoposide
|
 |
 |
0.4 mg/ml |
20-23°C |
 |
| 7 |
 |
|
2167 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
23-27°C |
 |
| 21 |
 |
|
4369 |
 Etoposide Etoposide
|
 |
 |
0,6 mg/ml |
23-27°C |
 |
| 21 |
 |
|
4369 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
25°C |
 |
| 21 |
 |
|
4024 |
 Etoposide Etoposide
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 21 |
 |
|
4024 |
 Etoposide Etoposide
|
 |
 |
0,4 mg/ml |
25°C |
 |
| 21 |
 |
|
4024 |
 Etoposide Etoposide
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 21 |
 |
|
4024 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
10 & 20 mg/ml |
25°C |
 |
| 96 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
10 & 20 mg/ml |
37°C |
 |
| 48 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
> 0,1 mg/ml |
25°C |
 |
| 96 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
> 0,1 mg/ml |
37°C |
 |
| 48 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
10 & 20 mg/ml |
25°C |
 |
| 96 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
10 & 20 mg/ml |
37°C |
 |
| 48 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
> 0,1 mg/ml |
25°C |
 |
| 96 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
> 0,1 mg/ml |
37°C |
 |
| 48 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
> 0,1 mg/ml |
25°C |
 |
| 96 |
 |
|
3655 |
 Etoposide phosphate Etoposide phosphate
|
 |
 |
> 0,1 mg/ml |
37°C |
 |
| 48 |
 |
|
3655 |
 Faricimab Faricimab
|
 |
 |
120 mg/ml |
4°C |
 |
| 37 |
 |
|
4790 |
 Fenoldopam mesylate Fenoldopam mesylate
|
 |
 |
4 >> 300 µg/ml |
23°C |
 |
| 72 |
 |
|
1618 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
4,55 µg/ml |
22°C |
 |
| 48 |
 |
|
223 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
4,55 µg/ml |
22°C |
 |
| 48 |
 |
|
223 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
50 µg/ml |
22°C |
 |
| 28 |
 |
|
2031 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
10 µg/ml |
22°C |
 |
| 93 |
 |
|
3916 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
50 µg/ml |
22°C |
 |
| 93 |
 |
|
3916 |
 Fentanyl citrate Fentanyl citrate
|
 |
 |
50 µg/ml |
22°C |
 |
| 28 |
 |
|
2031 |
 Fluconazole Fluconazole
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
257 |
 Fluconazole Fluconazole
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
257 |
 Fludarabine phosphate Fludarabine phosphate
|
 |
 |
0,5 mg/ml |
20-25°C |
 |
| 28 |
 |
|
2344 |
 Fluorouracil Fluorouracil
|
 |
 |
0,5 & 5 mg/ml |
25°C |
 |
| 13 |
 |
|
39 |
 Fluorouracil Fluorouracil
|
 |
 |
1 & 10 mg/ml |
21°C |
 |
| 14 |
 |
|
1475 |
 Fluorouracil Fluorouracil
|
 |
 |
10 mg/ml |
25°C |
 |
| 112 |
 |
|
1040 |
 Fluorouracil Fluorouracil
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 7 |
 |
|
1812 |
 Fluorouracil Fluorouracil
|
 |
 |
35 mg/ml |
21-25°C |
 |
| 28 |
 |
|
4568 |
 Fluorouracil Fluorouracil
|
 |
 |
35 mg/ml |
30°C |
 |
| 3 |
 |
|
4568 |
 Fluorouracil Fluorouracil
|
 |
 |
44 mg/ml |
21-25°C |
 |
| 28 |
 |
|
4568 |
 Fluorouracil Fluorouracil
|
 |
 |
44 mg/ml |
30°C |
 |
| 3 |
 |
|
4568 |
 Fosaprepitant dimeglumine Fosaprepitant dimeglumine
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
3459 |
 Fosphenytoin sodium Fosphenytoin sodium
|
 |
 |
50 mg/ml |
-20°C |
 |
| 30 |
 |
|
800 |
 Furosemide Furosemide
|
 |
 |
1,2 > 3,2 mg/ml |
22°C |
 |
| 7 |
 |
|
1680 |
 Furosemide Furosemide
|
 |
 |
1,0 > 8,0 mg/ml |
22°C |
 |
| 7 |
 |
|
1680 |
 Furosemide Furosemide
|
 |
 |
5 mg/ml |
20°C |
 |
| 24 |
 |
|
4341 |
 Ganciclovir sodium Ganciclovir sodium
|
 |
 |
5,8 mg/ml |
25°C |
 |
| 12 |
 |
|
74 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
2 mg/ml |
25°C |
 |
| 28 |
 |
|
3496 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
5.2 mg/ml |
25°C |
 |
| 28 |
 |
|
3496 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
2 mg/ml |
25°C |
 |
| 28 |
 |
|
3496 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
5.2 mg/ml |
25°C |
 |
| 28 |
 |
|
3496 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
0,1 & 25 (Ebegemcit® Lyo) mg/ml |
20-25°C |
 |
| 14 |
 |
|
2344 |
 Gemcitabine hydrochloride Gemcitabine hydrochloride
|
 |
 |
0,1 & 25 (Ebegemcit® Lyo) mg/ml |
20-25°C |
 |
| 14 |
 |
|
2344 |
 Gentamicin sulfate Gentamicin sulfate
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
434 |
 Gentamicin sulfate Gentamicin sulfate
|
 |
 |
1 mg/ml |
25°C |
 |
| 48 |
 |
|
434 |
 Gentamicin sulfate Gentamicin sulfate
|
 |
 |
30 mg/ml |
22°C-23°C |
 |
| 48 |
 |
|
639 |
 Glycopyrronium bromide Glycopyrronium bromide
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 90 |
 |
|
1756 |
 Heparin sodium Heparin sodium
|
 |
 |
1 UI/ml |
25°C |
 |
| 365 |
 |
|
4166 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
1 mg/ml |
25°C |
 |
| 3 |
 |
|
739 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
1 mg/ml |
25°C |
 |
| 3 |
 |
|
739 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
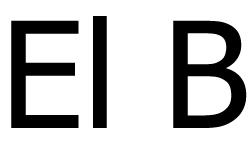 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
739 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
1 mg/ml |
25°C |
 |
| 2 |
 |
|
2001 |
 Hydrocortisone sodium succinate Hydrocortisone sodium succinate
|
 |
 |
50 mg/ml |
25°C |
 |
| 15 |
 |
|
2001 |
 Hydromorphone hydrochloride Hydromorphone hydrochloride
|
 |
 |
1,5 & 80 mg/ml |
23°C |
 |
| 60 |
 |
|
1706 |
 Ibuprofen lysinate Ibuprofen lysinate
|
 |
 |
1 & 4 mg/ml |
25°C |
 |
| 15 |
 |
|
1985 |
 Ibuprofen lysinate Ibuprofen lysinate
|
 |
 |
1 & 4 mg/ml |
25°C |
 |
| 15 |
 |
|
1985 |
 Indomethacin sodium trihydrate Indomethacin sodium trihydrate
|
 |
 |
0,5 mg/ml |
21°C-25°C |
 |
| 12 |
 |
|
303 |
 Indomethacin sodium trihydrate Indomethacin sodium trihydrate
|
 |
 |
0,5 mg/ml |
21°C-25°C |
 |
| 14 |
 |
|
303 |
 Infliximab Infliximab
|
 |
 |
0.6 & 0.84 & 1.88 mg/ml |
25°C |
 |
| 7 |
 |
|
3825 |
 Insulin Insulin
|
 |
 |
1 UI/ml |
20-25°C |
 |
| 48 |
 |
|
4635 |
 Ioxitalamate sodium Ioxitalamate sodium
|
 |
 |
0,12 mg I/ml |
25°C |
 |
| 90 |
 |
|
1809 |
 Isoniazid Isoniazid
|
 |
 |
mg/ml |
25°C |
 |
| 42 |
 |
|
2837 |
 Ixabepilone Ixabepilone
|
 |
 |
0,2 >> 0,6 mg/ml |
25°C |
 |
| 6 |
 |
|
3084 |
 Ixabepilone Ixabepilone
|
 |
 |
0,2 >> 0,6 mg/ml |
25°C |
 |
| 6 |
 |
|
3084 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
10 mg/ml |
25°C |
 |
| 182 |
 |
|
3435 |
 Ketamine hydrochloride Ketamine hydrochloride
|
 |
 |
0,1 mg/ml |
21°C |
 |
| 24 |
 |
|
1864 |
 Ketoprofene Ketoprofene
|
 |
 |
0,96 mg/ml |
20-24°C |
 |
| 24 |
 |
|
3075 |
 Ketorolac tromethamine Ketorolac tromethamine
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 48 |
 |
|
469 |
 Ketorolac tromethamine Ketorolac tromethamine
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 48 |
 |
|
469 |
 Ketorolac tromethamine Ketorolac tromethamine
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 48 |
 |
|
469 |
 Ketorolac tromethamine Ketorolac tromethamine
|
 |
 |
0,1 & 0,3 mg/ml |
4°C |
 |
| 35 |
 |
|
2124 |
 Lansoprazole Lansoprazole
|
 |
 |
mg/ml |
25°C |
 |
| 91 |
 |
|
2594 |
 Lansoprazole Lansoprazole
|
 |
 |
mg/ml |
25°C |
 |
| 91 |
 |
|
2594 |
 Lansoprazole Lansoprazole
|
 |
 |
mg/ml |
22°C |
 |
| 8 |
 |
|
2546 |
 Levofolinate disodium Levofolinate disodium
|
 |
 |
1,8 mg/ml |
20-25°C |
 |
| 72 |
 |
|
3369 |
 Levofolinate disodium Levofolinate disodium
|
 |
 |
3,6 mg/ml |
20-25°C |
 |
| 72 |
 |
|
3369 |
 Levosimendan Levosimendan
|
 |
 |
2,5 mg/ml |
8°C |
 |
| 60 |
 |
|
3417 |
 Levothyroxine Levothyroxine
|
 |
 |
0,4 µg/ml |
25°C |
 |
| 16 |
 |
|
3166 |
 Levothyroxine Levothyroxine
|
 |
 |
2 µg/ml |
25°C |
 |
| 6 |
 |
|
3166 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
2 mg/ml |
25°C |
 |
| 14 |
 |
|
619 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
2 mg/ml |
25°C |
 |
| 14 |
 |
|
619 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
2 mg/ml |
25°C |
 |
| 14 |
 |
|
619 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
2 mg/ml |
25°C |
 |
| 14 |
 |
|
619 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
20 mg/ml |
25°C |
 |
| 90 |
 |
|
1715 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
20 mg/ml |
25°C |
 |
| 90 |
 |
|
1715 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
mg/mL |
21°C |
 |
| 21 |
 |
|
3978 |
 Lidocaine hydrochloride Lidocaine hydrochloride
|
 |
 |
mg/mL |
21°C |
 |
| 21 |
 |
|
3978 |
 Lincomycin Lincomycin
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 31 |
 |
|
4118 |
 Lincomycin Lincomycin
|
 |
 |
0,6 mg/ml |
25°C |
 |
| 31 |
 |
|
4118 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
25°C |
 |
| 28 |
 |
|
2389 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
25°C |
 |
| 90 |
 |
|
2549 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
25°C |
 |
| 56 |
 |
|
2549 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
4°C |
 |
| 90 |
 |
|
2549 |
 Lisinopril Lisinopril
|
 |
 |
mg/ml |
4°C |
 |
| 56 |
 |
|
2549 |
 Lorazepam Lorazepam
|
 |
 |
1 & 2 mg/ml |
22°C |
 |
| 28 |
 |
|
765 |
 Lorazepam Lorazepam
|
 |
 |
0,2 mg/ml |
25°C |
 |
| 24 |
 |
|
2146 |
 Lorazepam Lorazepam
|
 |
 |
1 mg/ml |
22°C |
 |
| 28 |
 |
|
765 |
 Maprotiline Maprotiline
|
 |
 |
? mg/ml |
25°C |
 |
| 4 |
 |
|
54 |
 Maprotiline Maprotiline
|
 |
 |
? mg/ml |
25°C |
 |
| 4 |
 |
|
54 |
 Melphalan Melphalan
|
 |
 |
0,5 mg/ml |
23-27°C |
 |
| 2 |
 |
|
3977 |
 Melphalan Melphalan
|
 |
 |
2 mg/ml |
23-27°C |
 |
| 6 |
 |
|
3977 |
 Melphalan Melphalan
|
 |
 |
4 mg/ml |
23-27°C |
 |
| 8 |
 |
|
3977 |
 Melphalan Melphalan
|
 |
 |
0,2 mg/ml |
20°C |
 |
| 12 |
 |
|
160 |
 Melphalan Melphalan
|
 |
 |
0,2 mg/ml |
23°C |
 |
| 5 |
 |
|
160 |
 Melphalan Melphalan
|
 |
 |
0,2 mg/ml |
26°C |
 |
| 3 |
 |
|
160 |
 Melphalan Melphalan
|
 |
 |
2 mg/ml |
23°C |
 |
| 4 |
 |
|
160 |
 Melphalan Melphalan
|
 |
 |
2 mg/ml |
26°C |
 |
| 3 |
 |
|
160 |
 Melphalan Melphalan
|
 |
 |
2 mg/ml |
4°C |
 |
| 4 |
 |
|
3516 |
 Meropenem Meropenem
|
 |
 |
10 mg/ml |
25°C |
 |
| 8 |
 |
|
4326 |
 Meropenem Meropenem
|
 |
 |
20 mg/ml |
25°C |
 |
| 8 |
 |
|
4326 |
 Meropenem Meropenem
|
 |
 |
40 mg/ml |
25°C |
 |
| 4 |
 |
|
4326 |
 Meropenem Meropenem
|
 |
 |
41,7 mg/ml |
20-25°C |
 |
| 8 |
 |
|
4634 |
 Meropenem Meropenem
|
 |
 |
41,7 mg/ml |
20-25°C |
 |
| 4 |
 |
|
4634 |
 Metamizol sodium Metamizol sodium
|
 |
 |
1 & 60 mg/ml |
25°C |
 |
| 24 |
 |
|
1685 |
 Metaraminol Metaraminol
|
 |
 |
0,5 mg/ml |
25°C |
 |
| 378 |
 |
|
3758 |
 Methadone hydrochloride Methadone hydrochloride
|
 |
 |
1 & 2 & 5 mg/ml |
25°C |
 |
| 28 |
 |
|
224 |
 Methotrexate sodium Methotrexate sodium
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 7 |
 |
|
739 |
 Methotrexate sodium Methotrexate sodium
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 7 |
 |
|
739 |
 Methotrexate sodium Methotrexate sodium
|
 |
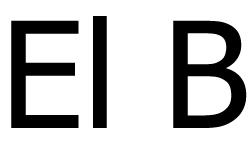 |
2,5 mg/ml |
25°C |
 |
| 7 |
 |
|
739 |
 Metoprolol tartrate Metoprolol tartrate
|
 |
 |
0,4 mg/ml |
24°C |
 |
| 36 |
 |
|
238 |
 Midazolam hydrochloride Midazolam hydrochloride
|
 |
 |
3 mg/ml |
20°C |
 |
| 7 |
 |
|
401 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,4 & 0,8 mg/ml |
20-25°C |
 |
| 14 |
 |
|
928 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,4 mg/ml |
22°C-23°C |
 |
| 7 |
 |
|
813 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,4 mg/ml |
22°C-23°C |
 |
| 7 |
 |
|
813 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,4 mg/ml |
22°C-23°C |
 |
| 7 |
 |
|
813 |
 Milrinone lactate Milrinone lactate
|
 |
 |
0,4 mg/ml |
22°C-23°C |
 |
| 7 |
 |
|
813 |
 Mitoxantrone dihydrochloride Mitoxantrone dihydrochloride
|
 |
 |
0,3 >> 0,5 mg/ml |
20-25°C |
 |
| 28 |
 |
|
2344 |
 Mitoxantrone dihydrochloride Mitoxantrone dihydrochloride
|
 |
 |
0,04 >> 0,5 mg/ml |
20-25°C |
 |
| 7 |
 |
|
2344 |
 Mitoxantrone dihydrochloride Mitoxantrone dihydrochloride
|
 |
 |
0,3 >> 0,5 mg/ml |
20-25°C |
 |
| 28 |
 |
|
2344 |
 Mitoxantrone dihydrochloride Mitoxantrone dihydrochloride
|
 |
 |
0,04 >> 0,5 mg/ml |
20-25°C |
 |
| 7 |
 |
|
2344 |
 Mitoxantrone dihydrochloride Mitoxantrone dihydrochloride
|
 |
 |
0,3 >> 0,5 mg/ml |
20-25°C |
 |
| 28 |
 |
|
2344 |
 Morphine hydrochloride Morphine hydrochloride
|
 |
 |
mg/g |
15-25°C |
 |
| 1095 |
 |
|
4653 |
 Morphine sulfate Morphine sulfate
|
 |
 |
1,2 mg/ml |
21°C |
 |
| 36 |
 |
|
340 |
 Morphine sulfate Morphine sulfate
|
 |
 |
10 mg/ml |
23°C |
 |
| 30 |
 |
|
120 |
 Morphine sulfate Morphine sulfate
|
 |
 |
1,76 mg/ml |
20°C-25°C |
 |
| 14 |
 |
|
1968 |
 Morphine sulfate Morphine sulfate
|
 |
 |
5 & 50 mg/ml |
23°C |
 |
| 60 |
 |
|
1709 |
 Morphine sulfate Morphine sulfate
|
 |
 |
mg |
25°C |
 |
| 22 |
 |
|
2828 |
 Mycophenolate mofetil Mycophenolate mofetil
|
 |
 |
mg/ml |
21-28°C |
 |
| 28 |
 |
|
2543 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
60 mg/ml |
23-27°C |
 |
| 72 |
 |
|
4639 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
60 mg/ml |
23-27°C |
 |
| 72 |
 |
|
4639 |
 N-acetylcysteine N-acetylcysteine
|
 |
 |
60 mg/ml |
23-27°C |
 |
| 72 |
 |
|
4639 |
 Nefopam Nefopam
|
 |
 |
0,19 mg/ml |
20-24°C |
 |
| 24 |
 |
|
3075 |
 Nefopam Nefopam
|
 |
 |
2,5 mg/ml |
20-25°C |
 |
| 48 |
 |
|
4267 |
 Neostigmine methylsulfate Neostigmine methylsulfate
|
 |
 |
1 mg/ml |
25°C |
 |
| 90 |
 |
|
1682 |
 Netilmicin sulfate Netilmicin sulfate
|
 |
 |
3 mg/ml |
24°C |
 |
| 24 |
 |
|
421 |
 Netilmicin sulfate Netilmicin sulfate
|
 |
 |
3 mg/ml |
24°C |
 |
| 24 |
 |
|
421 |
 Nicardipine hydrochloride Nicardipine hydrochloride
|
 |
 |
0,05 & 0,5 mg/ml |
18°C-28°C |
 |
| 7 |
 |
|
44 |
 Nicardipine hydrochloride Nicardipine hydrochloride
|
 |
 |
0,05 & 0,5 mg/ml |
18°C-28°C |
 |
| 7 |
 |
|
44 |
 Nimustine Nimustine
|
 |
 |
2 mg/ml |
25°C |
 |
| 5 |
 |
|
525 |
 Nimustine Nimustine
|
 |
 |
2 mg/ml |
25°C |
 |
| 7 |
 |
|
525 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,05 mg/ml |
35-37°C |
 |
| 48 |
 |
|
708 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,09 mg/ml |
25°C |
 |
| 24 |
 |
|
621 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,2 & 0,4 mg/ml |
18-27°C |
 |
| 28 |
 |
|
1027 |
 Nitroglycerin Nitroglycerin
|
 |
 |
1 mg/ml |
23°C |
 |
| 24 |
 |
|
192 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,2 & 0,4 mg/ml |
18-27°C |
 |
| 28 |
 |
|
1027 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,2 & 0,4 mg/ml |
18-27°C |
 |
| 28 |
 |
|
1027 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,06 mg/ml |
20-25°C |
 |
| 48 |
 |
|
1960 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,09 mg/ml |
25°C |
 |
| 24 |
 |
|
621 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,1 mg/ml |
25°C |
 |
| 35 |
 |
|
1980 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,06 mg/ml |
25°C |
 |
| 48 |
 |
|
1522 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,2 & 0,4 mg/ml |
18-27°C |
 |
| 28 |
 |
|
1027 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,42 mg/ml |
25°C |
 |
| 24 |
 |
|
2146 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,2 & 0,4 mg/ml |
18-27°C |
 |
| 28 |
 |
|
1027 |
 Nitroglycerin Nitroglycerin
|
 |
 |
0,2 & 0,4 mg/ml |
18-27°C |
 |
| 28 |
 |
|
1027 |
 Nitroglycerin Nitroglycerin
|
 |
 |
1 mg/ml |
25°C |
 |
| 24 |
 |
|
196 |
 Nivolumab Nivolumab
|
 |
 |
1 >> 10 mg/ml |
25°C |
 |
| 24 |
 |
|
4652 |
 Nivolumab Nivolumab
|
 |
 |
1 >> 10 mg/ml |
25°C |
 |
| 8 |
 |
|
4652 |
 Nivolumab Nivolumab
|
 |
 |
10 mg/ml |
25°C |
 |
| 48 |
 |
|
4652 |
 Nivolumab Nivolumab
|
 |
 |
1 >> 10 mg/ml |
25°C |
 |
| 24 |
 |
|
4652 |
 Nivolumab Nivolumab
|
 |
 |
1 >> 10 mg/ml |
25°C |
 |
| 8 |
 |
|
4652 |
 Nivolumab Nivolumab
|
 |
 |
1 >> 10 mg/ml |
25°C |
 |
| 24 |
 |
|
4652 |
 Nivolumab Nivolumab
|
 |
 |
1 >> 10 mg/ml |
25°C |
 |
| 8 |
 |
|
4652 |
 Nizatidine Nizatidine
|
 |
 |
0,75 & 1,5 & 3 mg/ml |
25°C |
 |
| 7 |
 |
|
388 |
 Nizatidine Nizatidine
|
 |
 |
0,75 & 1,5 & 3 mg/ml |
25°C |
 |
| 7 |
 |
|
388 |
 Nizatidine Nizatidine
|
 |
 |
0,75 & 1,5 & 3 mg/ml |
25°C |
 |
| 7 |
 |
|
388 |
 Nizatidine Nizatidine
|
 |
 |
0,75 & 1,5 & 3 mg/ml |
25°C |
 |
| 7 |
 |
|
388 |
 Nizatidine Nizatidine
|
 |
 |
0,75 & 3 mg/ml |
25°C |
 |
| 7 |
 |
|
388 |
 Nizatidine Nizatidine
|
 |
 |
0,75 & 3 mg/ml |
25°C |
 |
| 7 |
 |
|
388 |
 Norepinephrine bitartrate Norepinephrine bitartrate
|
 |
 |
0,004 & 0,008 mg/ml |
25°C |
 |
| 24 |
 |
|
642 |
 Omacetaxine mepesuccinate Omacetaxine mepesuccinate
|
 |
 |
3,5 mg/ml |
2-8°C |
 |
| 14 |
 |
|
4123 |
 Omacetaxine mepesuccinate Omacetaxine mepesuccinate
|
 |
 |
3,5 mg/ml |
25°C |
 |
| 24 |
 |
|
4123 |
 Omeprazole sodium Omeprazole sodium
|
 |
 |
mg/ml |
22°C |
 |
| 14 |
 |
|
2546 |
 Omeprazole sodium Omeprazole sodium
|
 |
 |
0,4 mg/ml |
22°C |
 |
| 48 |
 |
|
4316 |
 Ondansetron hydrochloride Ondansetron hydrochloride
|
 |
 |
0,024 & 0,096 mg/ml |
21-26°C |
 |
| 14 |
 |
|
333 |
 Ondansetron hydrochloride Ondansetron hydrochloride
|
 |
 |
0,03 & 0,3 mg/ml |
25°C |
 |
| 48 |
 |
|
183 |
 Ondansetron hydrochloride Ondansetron hydrochloride
|
 |
 |
0,25 & 0,5 & 1 mg/ml |
22-25°C |
 |
| 48 |
 |
|
915 |
 Ondansetron hydrochloride Ondansetron hydrochloride
|
 |
 |
2 mg/ml |
22-25°C |
 |
| 48 |
 |
|
915 |
 Ondansetron hydrochloride Ondansetron hydrochloride
|
 |
 |
0,03 & 0,3 mg/ml |
4°C |
 |
| 14 |
 |
|
113 |
 Ornidazole Ornidazole
|
 |
 |
10 mg/ml |
25°C |
 |
| 15 |
 |
|
918 |
 Ornidazole Ornidazole
|
 |
 |
4 mg/ml |
25°C |
 |
| 30 |
 |
|
918 |
 Ornidazole Ornidazole
|
 |
 |
10 mg/ml |
25°C |
 |
| 30 |
 |
|
918 |
 Ornidazole Ornidazole
|
 |
 |
4 mg/ml |
25°C |
 |
| 15 |
 |
|
918 |
 Oxaliplatin Oxaliplatin
|
 |
 |
5 mg/ml |
25°C |
 |
| 28 |
 |
|
2258 |
 Oxaliplatin Oxaliplatin
|
 |
 |
0.2 & 1 mg/ml |
25°C |
 |
| 8 |
 |
|
2259 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
5 & 50 mg/ml |
24°C |
 |
| 35 |
 |
|
1726 |
 Oxycodone hydrochloride Oxycodone hydrochloride
|
 |
 |
100 mg/ml |
24°C |
 |
| 35 |
 |
|
1726 |
 Oxytocin Oxytocin
|
 |
 |
0.02 UI/ml |
22-25°C |
 |
| 21 |
 |
|
3347 |
 Paclitaxel Paclitaxel
|
 |
 |
0,4 & 1,2 mg/ml |
20°C-25°C |
 |
| 5 |
 |
|
1865 |
 Paclitaxel Paclitaxel
|
 |
 |
0,3 & 1,2 mg/ml |
20°C-23°C |
 |
| 48 |
 |
|
669 |
 Paclitaxel Paclitaxel
|
 |
 |
0.3 > 1.2 mg/ml |
25°C |
 |
| 72 |
 |
|
1956 |
 Paclitaxel Paclitaxel
|
 |
 |
0.3 > 1.2 mg/ml |
25°C |
 |
| 72 |
 |
|
1956 |
 Palonosetron hydrochloride Palonosetron hydrochloride
|
 |
 |
5 & 30 µg/ml |
23°C |
 |
| 48 |
 |
|
1936 |
 Pantoprazole sodium Pantoprazole sodium
|
 |
 |
4 mg/ml |
20-25°C |
 |
| 3 |
 |
|
3220 |
 Pantoprazole sodium Pantoprazole sodium
|
 |
 |
0,4 & 0,8 mg/ml |
20-25°C |
 |
| 3 |
 |
|
3220 |
 Pantoprazole sodium Pantoprazole sodium
|
 |
 |
0,4 mg/ml |
20-25°C |
 |
| 2 |
 |
|
3220 |
 Pantoprazole sodium Pantoprazole sodium
|
 |
 |
0,8 mg/ml |
20-25°C |
 |
| 3 |
 |
|
3220 |
 Pantoprazole sodium Pantoprazole sodium
|
 |
 |
4 mg/ml |
23°C-25°C |
 |
| 48 |
 |
|
2030 |
 Pantoprazole sodium Pantoprazole sodium
|
 |
 |
0,4 mg/ml |
22°C |
 |
| 48 |
 |
|
4316 |
 Parecoxib sodium Parecoxib sodium
|
 |
 |
16 & 20 mg/ml |
25°C |
 |
| 48 |
 |
|
1824 |
 Parecoxib sodium Parecoxib sodium
|
 |
 |
16 & 20 mg/ml |
25°C |
 |
| 48 |
 |
|
1824 |
 Parecoxib sodium Parecoxib sodium
|
 |
 |
16 & 20 mg/ml |
25°C |
 |
| 48 |
 |
|
1824 |
 Parecoxib sodium Parecoxib sodium
|
 |
 |
13,3 & 20 mg/ml |
25°C |
 |
| 48 |
 |
|
1824 |
 Parecoxib sodium Parecoxib sodium
|
 |
 |
13,3 & 20 mg/ml |
25°C |
 |
| 48 |
 |
|
1824 |
 Parecoxib sodium Parecoxib sodium
|
 |
 |
13,3 & 20 mg/ml |
25°C |
 |
| 48 |
 |
|
1824 |
 Pembrolizumab Pembrolizumab
|
 |
 |
1 mg/ml |
17-23°C |
 |
| 7 |
 |
|
4334 |
 Pembrolizumab Pembrolizumab
|
 |
 |
1 mg/ml |
2-8°C |
 |
| 7 |
 |
|
4334 |
 Pemetrexed diarginine Pemetrexed diarginine
|
 |
 |
25 mg/ml |
22-25°C |
 |
| 1 |
 |
|
4546 |
 Pemetrexed diarginine Pemetrexed diarginine
|
 |
 |
25 mg/ml |
25°C |
 |
| 4 |
 |
|
4702 |
 Pemetrexed diarginine Pemetrexed diarginine
|
 |
 |
12 mg/ml |
22-25°C |
 |
| 1 |
 |
|
4546 |
 Pemetrexed diarginine Pemetrexed diarginine
|
 |
 |
4,9 mg/ml |
22-25°C |
 |
| 1 |
 |
|
4546 |
 Pentamidine isetionate Pentamidine isetionate
|
 |
 |
1 & 2 mg/ml |
22°C-26°C |
 |
| 48 |
 |
|
424 |
 Pertuzumab Pertuzumab
|
 |
 |
3 mg/ml |
30°C |
 |
| 24 |
 |
|
3366 |
 Pethidine hydrochloride Pethidine hydrochloride
|
 |
 |
2,5 mg/ml |
25°C |
 |
| 24 |
 |
|
1292 |
 Pethidine hydrochloride Pethidine hydrochloride
|
 |
 |
1,2 mg/ml |
21°C |
 |
| 36 |
 |
|
340 |
 Pethidine hydrochloride Pethidine hydrochloride
|
 |
 |
10 mg/ml |
23°C |
 |
| 84 |
 |
|
119 |
 Phenylephrine hydrochloride Phenylephrine hydrochloride
|
 |
 |
0,1 mg/ml |
23-25°C |
 |
| 30 |
 |
|
2194 |
 Piperacillin sodium Piperacillin sodium
|
 |
 |
40 mg/ml |
25°C |
 |
| 48 |
 |
|
432 |
 Piperacillin sodium Piperacillin sodium
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4634 |
 Piperacillin sodium Piperacillin sodium
|
 |
 |
125 mg/ml |
20-25°C |
 |
| 48 |
 |
|
4634 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
40 / 5 mg/ml |
25°C |
 |
| 4 |
 |
|
2026 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
40 / 5 mg/ml |
25°C |
 |
| 3 |
 |
|
2026 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
125/15,6 mg/ml |
20-25°C |
 |
| 48 |
 |
|
4634 |
 Piperacillin sodium / tazobactam Piperacillin sodium / tazobactam
|
 |
 |
80 / 10 mg/ml |
25°C |
 |
| 12 |
 |
|
4326 |
 Prednisolone Prednisolone
|
 |
 |
mg/ml |
22-24°C |
 |
| 365 |
 |
|
2557 |
 Propafenone hydrochloride Propafenone hydrochloride
|
 |
 |
0,5 & 2 mg/ml |
20°C-22°C |
 |
| 48 |
 |
|
193 |
 Propafenone hydrochloride Propafenone hydrochloride
|
 |
 |
0,5 & 2 mg/ml |
20°C-22°C |
 |
| 48 |
 |
|
193 |
 Propranolol hydrochloride Propranolol hydrochloride
|
 |
 |
mg/ml |
25°C |
 |
| 120 |
 |
|
2477 |
 Propranolol hydrochloride Propranolol hydrochloride
|
 |
 |
mg/ml |
2°C |
 |
| 120 |
 |
|
2477 |
 Propranolol hydrochloride Propranolol hydrochloride
|
 |
 |
0,0005 & 0,02 mg/ml |
25°C |
 |
| 24 |
 |
|
1198 |
 Propranolol hydrochloride Propranolol hydrochloride
|
 |
 |
0,0005 & 0,02 mg/ml |
25°C |
 |
| 24 |
 |
|
1198 |
 Propranolol hydrochloride Propranolol hydrochloride
|
 |
 |
0,0005 & 0,02 mg/ml |
25°C |
 |
| 24 |
 |
|
1198 |
 Quinine dihydrochloride Quinine dihydrochloride
|
 |
 |
1,2 mg/ml |
27°C |
 |
| 24 |
 |
|
916 |
 Raltitrexed Raltitrexed
|
 |
 |
0,5 mg/ml |
25°C |
 |
| 24 |
 |
|
1518 |
 Raltitrexed Raltitrexed
|
 |
 |
? mg/ml |
25°C |
 |
| 24 |
 |
|
1518 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
0,05 mg/ml |
20°C-25°C |
 |
| 7 |
 |
|
213 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
0,5 & 1 & 2 mg/ml |
25°C |
 |
| 7 |
 |
|
78 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
0,5 >> 2 mg/ml |
20°C-25°C |
 |
| 28 |
 |
|
213 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
0,05 mg/ml |
20°C-25°C |
 |
| 2 |
 |
|
213 |
 Ranitidine hydrochloride Ranitidine hydrochloride
|
 |
 |
0,5 & 1 & 2 mg/ml |
25°C |
 |
| 7 |
 |
|
78 |
 Ribavirine Ribavirine
|
 |
 |
mg |
25°C |
 |
| 45 |
 |
|
3917 |
 Ribavirine Ribavirine
|
 |
 |
mg |
4°C |
 |
| 45 |
 |
|
3917 |
 Ribavirine Ribavirine
|
 |
 |
mg |
25°C |
 |
| 45 |
 |
|
3917 |
 Ribavirine Ribavirine
|
 |
 |
mg |
4°C |
 |
| 45 |
 |
|
3917 |
 Rituximab Rituximab
|
 |
 |
120 mg/ml |
30°C |
 |
| 24 |
 |
|
2825 |
 Scopolamine hydrobromide Scopolamine hydrobromide
|
 |
 |
mg |
25°C |
 |
| 42 |
 |
|
2876 |
 Sildenafil citrate Sildenafil citrate
|
 |
 |
0,4 mg/ml |
20°C |
 |
| 30 |
 |
|
3923 |
 Sodium bicarbonate Sodium bicarbonate
|
 |
 |
4 mg/ml |
21-24°C |
 |
| 48 |
 |
|
3116 |
 Sodium bicarbonate Sodium bicarbonate
|
 |
 |
12,6 mg/ml |
23-25°C |
 |
| 7 |
 |
|
3329 |
 Sodium bicarbonate Sodium bicarbonate
|
 |
 |
7,6 >> 11,45 mg/ml |
21-24°C |
 |
| 30 |
 |
|
3116 |
 Sodium bicarbonate Sodium bicarbonate
|
 |
 |
8,4 mg/ml |
23-25°C |
 |
| 7 |
 |
|
3329 |
 Sodium Phosphate Sodium Phosphate
|
 |
 |
0,030 mmol/ml |
23°C |
 |
| 63 |
 |
|
3945 |
 Sodium Phosphate Sodium Phosphate
|
 |
 |
0,030 mmol/ml |
4°C |
 |
| 63 |
 |
|
3945 |
 Sodium Phosphate Sodium Phosphate
|
 |
 |
0,150 mmol/ml |
23°C |
 |
| 63 |
 |
|
3945 |
 Sodium Phosphate Sodium Phosphate
|
 |
 |
0,150 mmol/ml |
4°C |
 |
| 63 |
 |
|
3945 |
 Sodium thiosulfate Sodium thiosulfate
|
 |
 |
16 mg/ml |
25°C |
 |
| 24 |
 |
|
4126 |
 Sodium thiosulfate Sodium thiosulfate
|
 |
 |
72 mg/ml |
25°C |
 |
| 24 |
 |
|
4126 |
 Spironolactone Spironolactone
|
 |
 |
mg/ml |
20-24°C |
 |
| 28 |
 |
|
2489 |
 Spironolactone Spironolactone
|
 |
 |
mg/ml |
20-24°C |
 |
| 28 |
 |
|
2489 |
 Spironolactone Spironolactone
|
 |
 |
mg/ml |
20-24°C |
 |
| 28 |
 |
|
2489 |
 Sufentanil citrate Sufentanil citrate
|
 |
 |
0.005 mg/ml |
25°C |
 |
| 24 |
 |
|
2146 |
 Suxamethonium chloride Suxamethonium chloride
|
 |
 |
20 mg/ml |
25°C |
 |
| 45 |
 |
|
1755 |
 Tacrolimus Tacrolimus
|
 |
 |
2 >> 8 µg/ml |
23°C |
 |
| 9 |
 |
|
3915 |
 Teicoplanine Teicoplanine
|
 |
 |
0,025 mg/ml |
25°C |
 |
| 32 |
 |
|
2450 |
 Teicoplanine Teicoplanine
|
 |
 |
0,025 mg/ml |
37°C |
 |
| 8 |
 |
|
2450 |
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
15 mg/ml |
20-25°C |
 |
| 12 |
 |
|
3839 |
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
15 mg/ml |
20-25°C |
 |
| 12 |
 |
|
3839 |
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
0.6 mg/ml |
20-25°C |
 |
| 12 |
 |
|
3839 |
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
8 mg/ml |
20-25°C |
 |
| 12 |
 |
|
3839 |
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
0.6 mg/ml |
20-25°C |
 |
| 12 |
 |
|
3839 |
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
8 mg/ml |
20-25°C |
 |
| 12 |
 |
|
3839 |
 Teniposide Teniposide
|
 |
 |
0,4 mg/ml |
20°C-22°C |
 |
| 4 |
 |
|
482 |
 Teniposide Teniposide
|
 |
 |
0,4 mg/ml |
20°C-22°C |
 |
| 4 |
 |
|
482 |
 Terbutaline sulfate Terbutaline sulfate
|
 |
 |
0,03 mg/ml |
25°C |
 |
| 7 |
 |
|
448 |
 Terbutaline sulfate Terbutaline sulfate
|
 |
 |
0,03 mg/ml |
25°C |
 |
| 7 |
 |
|
448 |
 Terbutaline sulfate Terbutaline sulfate
|
 |
 |
1 mg/ml |
25°C |
 |
| 7 |
 |
|
449 |
 Thiamazole Thiamazole
|
 |
 |
0,48 mg/ml |
25°C |
 |
| 24 |
 |
|
4518 |
 Thiamazole Thiamazole
|
 |
 |
0,96 mg/ml |
25°C |
 |
| 24 |
 |
|
4518 |
 Thiopental sodium Thiopental sodium
|
 |
 |
9 mg/ml |
25°C |
 |
| 24 |
 |
|
2146 |
 Thiotepa Thiotepa
|
 |
 |
10 mg/ml |
25°C |
 |
| 1 |
 |
|
4748 |
 Thiotepa Thiotepa
|
 |
 |
1 & 3 mg/ml |
25°C |
 |
| 24 |
 |
|
302 |
 Thiotepa Thiotepa
|
 |
 |
1 & 3 mg/ml |
8°C |
 |
| 48 |
 |
|
302 |
 Thiotepa Thiotepa
|
 |
 |
1 & 2 & 3 mg/ml |
25°C |
 |
| 1 |
 |
|
4748 |
 Thiotepa Thiotepa
|
 |
 |
1 mg/ml |
25°C |
 |
| 3 |
 |
|
4677 |
 Thiotepa Thiotepa
|
 |
 |
1 mg/ml |
25°C |
 |
| 3 |
 |
|
4748 |
 Thiotepa Thiotepa
|
 |
 |
2 mg/ml |
25°C |
 |
| 5 |
 |
|
4748 |
 Thiotepa Thiotepa
|
 |
 |
3 mg/ml |
25°C |
 |
| 5 |
 |
|
4677 |
 Thiotepa Thiotepa
|
 |
 |
3 mg/ml |
25°C |
 |
| 7 |
 |
|
4748 |
 Tiapride Tiapride
|
 |
 |
1 mg/ml |
23-27°C |
 |
| 48 |
 |
|
3384 |
 Tiapride Tiapride
|
 |
 |
2 mg/ml |
23-27°C |
 |
| 48 |
 |
|
3384 |
 Tirofiban Tirofiban
|
 |
 |
50 µg/ml |
20-25°C |
 |
| 30 |
 |
|
1600 |
 Tirofiban Tirofiban
|
 |
 |
50 µg/ml |
20°C-25°C |
 |
| 24 |
 |
|
1071 |
 Tirofiban Tirofiban
|
 |
 |
50 µg/ml |
20°C-25°C |
 |
| 24 |
 |
|
1071 |
 Tirofiban Tirofiban
|
 |
 |
50 µg/ml |
20-25°C |
 |
| 30 |
 |
|
1600 |
 Tobramycin sulfate Tobramycin sulfate
|
 |
 |
1,2 mg/ml |
25°C |
 |
| 48 |
 |
|
434 |
 Tobramycin sulfate Tobramycin sulfate
|
 |
 |
30 mg/ml |
22°C-23°C |
 |
| 48 |
 |
|
639 |
 Topotecan Topotecan
|
 |
 |
0,05 mg/ml |
23-24°C |
 |
| 24 |
 |
|
1075 |
 Topotecan Topotecan
|
 |
 |
0,025 mg/ml |
23°C-24°C |
 |
| 24 |
 |
|
1075 |
 Topotecan Topotecan
|
 |
 |
0,05 mg/ml |
23-24°C |
 |
| 24 |
 |
|
1075 |
 Topotecan Topotecan
|
 |
 |
0,01 mg/ml |
25°C |
 |
| 7 |
 |
|
1274 |
 Topotecan Topotecan
|
 |
 |
0,05 mg/ml |
23-24°C |
 |
| 24 |
 |
|
1075 |
 Torsemide Torsemide
|
 |
 |
0,2 & 5 mg/ml |
23-25°C |
 |
| 72 |
 |
|
818 |
 Tramadol hydrochloride Tramadol hydrochloride
|
 |
 |
0,89 mg/ml |
20-24°C |
 |
| 24 |
 |
|
3075 |
 Trastuzumab Trastuzumab
|
 |
 |
0,24 mg/ml |
30°C |
 |
| 24 |
 |
|
4163 |
 Trastuzumab Trastuzumab
|
 |
 |
3,84 mg/ml |
30°C |
 |
| 24 |
 |
|
4163 |
 Trastuzumab Trastuzumab
|
 |
 |
0,24 mg/ml |
30°C |
 |
| 24 |
 |
|
4163 |
 Trastuzumab Trastuzumab
|
 |
 |
3,84 mg/ml |
30°C |
 |
| 24 |
 |
|
4163 |
 Trastuzumab Trastuzumab
|
 |
 |
120 mg/ml |
<30°C |
 |
| 6 |
 |
|
3647 |
 Treosulfan Treosulfan
|
 |
 |
50 mg/ml |
25°C |
 |
| 4 |
 |
|
2197 |
 Treosulfan Treosulfan
|
 |
 |
20 mg/ml |
25°C |
 |
| 4 |
 |
|
2197 |
 Treosulfan Treosulfan
|
 |
 |
20 mg/ml |
25°C |
 |
| 4 |
 |
|
2197 |
 Treosulfan Treosulfan
|
 |
 |
20 mg/ml |
25°C |
 |
| 4 |
 |
|
2197 |
 Treprostinil Treprostinil
|
 |
 |
1 >> 10 mg/ml |
23°C |
 |
| 60 |
 |
|
1898 |
 Turoctocog alfa Turoctocog alfa
|
 |
 |
125 >> 750 UI/ml |
28-32°C |
 |
| 24 |
 |
|
4502 |
 Urokinase Urokinase
|
 |
 |
0,005 MUI/ml |
22-25°C |
 |
| 24 |
 |
|
375 |
 Urokinase Urokinase
|
 |
 |
0,005 MUI/ml |
22-25°C |
 |
| 24 |
 |
|
375 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
5 mg/ml |
22°C |
 |
| 48 |
 |
|
1671 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
0,025 mg/ml |
20°C |
 |
| 28 |
 |
|
2370 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
0,025 mg/ml |
37°C |
 |
| 5 |
 |
|
2370 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
40 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4007 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
41.67 mg/ml |
18-25°C |
 |
| 48 |
 |
|
4089 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
25 mg/ml |
23-27°C |
 |
| 24 |
 |
|
4373 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
40 mg/ml |
20-25°C |
 |
| 24 |
 |
|
4007 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
40 mg/ml |
23-27°C |
 |
| 24 |
 |
|
4373 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
62,5 mg/ml |
20-25°C |
 |
| 48 |
 |
|
4348 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
62,5 >> 83,3 mg/ml |
20-25°C |
 |
| 48 |
 |
|
4634 |
 Vancomycin hydrochloride Vancomycin hydrochloride
|
 |
 |
83,3 mg/ml |
20-25°C |
 |
| 48 |
 |
|
4348 |
 Vecuronium bromide Vecuronium bromide
|
 |
 |
0,04 mg/ml |
15-25°C |
 |
| 24 |
 |
|
3630 |
 Vecuronium bromide Vecuronium bromide
|
 |
 |
0,04 mg/ml |
15-25°C |
 |
| 24 |
 |
|
3630 |
 Vinorelbine tartrate Vinorelbine tartrate
|
 |
 |
0.5 & 3 mg/ml |
25°C |
 |
| 4 |
 |
|
2260 |
 Vinorelbine tartrate Vinorelbine tartrate
|
 |
 |
0,5 & 2 mg/ml |
23-25°C |
 |
| 5 |
 |
|
1413 |
 Vinorelbine tartrate Vinorelbine tartrate
|
 |
 |
0.5 & 3 mg/ml |
25°C |
 |
| 4 |
 |
|
2260 |
 Vinorelbine tartrate Vinorelbine tartrate
|
 |
 |
0.5 & 3 mg/ml |
25°C |
 |
| 4 |
 |
|
2260 |
 Voriconazole Voriconazole
|
 |
 |
2 mg/ml |
25°C |
 |
| 4 |
 |
|
4035 |
 Voriconazole Voriconazole
|
 |
 |
2 mg/ml |
35°C |
 |
| 4 |
 |
|
4035 |
 Voriconazole Voriconazole
|
 |
 |
2 mg/ml |
4°C |
 |
| 96 |
 |
|
4035 |
 Warfarin sodium Warfarin sodium
|
 |
 |
0,025 mg/ml |
21°C |
 |
| 5 |
 |
|
1472 |
 Warfarin sodium Warfarin sodium
|
 |
 |
0,025 mg/ml |
21°C |
 |
| 5 |
 |
|
1472 |
 Zonisamide Zonisamide
|
 |
 |
mg/ml |
23-25°C |
 |
| 7 |
 |
|
2383 |
 Zonisamide Zonisamide
|
 |
 |
mg/ml |
23-25°C |
 |
| 28 |
 |
|
2383 |
|