|
 |
Tradename |
|
|
Trade names are indicative and excipients composition can be different depending on the country and manufacturers
|
|
Amrizole |
Egypt, Saudi Arabia, United Arab Emirates |
|
Anaerobex |
Austria |
|
Bactrizol |
Venezuela |
|
Colpocin |
Greece |
|
Deflamon |
Italy |
|
Dumozol |
Egypt, Portugal |
|
Efloran |
Croatia |
|
Elyzol |
Saudi Arabia |
|
Emedal |
Greece |
|
Femagin |
Malaysia |
|
Flagyl |
Brazil, Chile, Colombia, Ecuador, Egypt, France, Greece, Iceland, Ireland, Luxembourg, Mexico, Morocco, Netherlands, Norway, Peru, Spain, Turkey, United States of America |
|
Flaxtec |
Mexico |
|
Flazol |
Egypt |
|
Flegyl |
Venezuela |
|
Gnostol |
Greece |
|
Klion |
Hungary |
|
Medazol |
Mexico |
|
Metrolag |
Switzerland, United Arab Emirates |
|
Metronidazole |
Australia, Belgium, Egypt, Finland, France, Great Britain, Greece, Iran, Ireland, Malaysia, Morocco, New Zealand, Norway, Portugal, Saudi Arabia, Sweden, Tunisia, United Arab Emirates |
|
Nidazol |
Iran, Saudi Arabia, Turkey, United Arab Emirates |
|
|
 |
 |
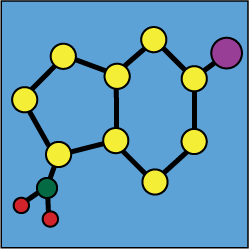
Stability of mixtures : Metronidazole |
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
2 mg/ml |
|
25°C |
| 20 |
 |
|
 |
630

|
 |
 |
2.6 mg/ml |
|
25°C |
| 4 |
 |
|
 |
2228

|
 |
 |
2,5 mg/ml |
|
23°C |
| 3 |
 |
|
 |
1988

|
 |
 |
5 mg/ml |
|
25°C |
| 2 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
25°C |
| 2 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
25°C |
| 2 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
25°C |
| 3 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
25°C |
| 7 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
25°C |
| 7 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
25°C |
| 22 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
5°C |
| 12 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
5°C |
| 12 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
5°C |
| 12 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
5°C |
| 12 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
5°C |
| 12 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
5°C |
| 12 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
5°C |
| 12 |
 |
|
 |
631

|
 |
 |
5 mg/ml |
|
8°C |
| 3 |
 |
|
 |
671

|
 |
 |
7,5 mg/ml |
|
24-26°C |
| 24 |
 |
|
 |
11

|
 |
 |
4 mg/ml |
|
25°C |
| 48 |
 |
|
 |
434
|
 |
 |
5 mg/ml |
|
28°C |
| 12 |
 |
|
 |
281

|
 |
 |
7,5 mg/ml |
|
4-6°C |
| 72 |
 |
|
 |
11

|
 |
 |
4 mg/ml |
|
4°C |
| 48 |
 |
|
 |
434
|
 |
 |
5 mg/ml |
|
5°C |
| 96 |
 |
|
 |
281

|
 |
 |
7,5 mg/ml |
|
24-26°C |
| 24 |
 |
|
 |
11

|
 |
 |
4 mg/ml |
|
25°C |
| 48 |
 |
|
 |
434
|
 |
 |
7,5 mg/ml |
|
4-6°C |
| 72 |
 |
|
 |
11

|
 |
 |
4 mg/ml |
|
4°C |
| 48 |
 |
|
 |
434
|
 |
 |
5 mg/ml |
|
22°C-24°C |
| 2 |
 |
|
 |
1605

|
 |
 |
5 mg/ml |
|
22°C-24°C |
| 4 |
 |
|
 |
1605

|
 |
 |
5 mg/ml |
|
22°C-24°C |
| 5 |
 |
|
|
1605

|
 |
 |
5 mg/ml |
|
25°C |
| 3 |
 |
|
 |
649

|
 |
 |
5 mg/ml |
|
25°C |
| 3 |
 |
|
 |
267

|
 |
 |
5 mg/ml |
|
4°C |
| 7 |
 |
|
 |
961

|
 |
 |
5 mg/ml |
|
4°C |
| 14 |
 |
|
 |
267

|
 |
 |
5 mg/ml |
|
4°C |
| 7 |
 |
|
 |
1605

|
 |
 |
5 mg/ml |
|
8°C |
| 72 |
 |
|
 |
282

|
 |
 |
5 mg/ml |
|
8°C |
| 72 |
 |
|
 |
282

|
 |
 |
5 mg/ml |
|
8°C |
| 72 |
 |
|
 |
282

|
 |
 |
4,1 mg/ml |
|
20°C |
| 4 |
 |
|
 |
1479

|
 |
 |
5 mg/ml |
|
25°C |
| 4 |
 |
|
 |
1692

|
|
|