|
 |
Tradename |
|
|
Trade names are indicative and excipients composition can be different depending on the country and manufacturers
|
|
Afipran |
Iceland, Norway |
|
Aristopramida |
Brazil |
|
Cerucal |
Hungary |
|
Clopan |
Ecuador |
|
Clopram |
Tunisia, United Arab Emirates |
|
Disgradon |
Argentina |
|
Doperan |
Tunisia |
|
Maxolon |
Australia, Great Britain, Ireland |
|
Meclomid |
Mexico |
|
Meclonir |
Colombia, Ecuador |
|
Meclop |
Egypt, Mexico |
|
Meclopram |
Egypt |
|
Metoclox |
Ecuador |
|
Metogastron |
Austria |
|
Metoplon |
Turkey |
|
Metpamid |
Turkey |
|
Paspertin |
Austria, Switzerland |
|
Plasil |
Brazil, Colombia, Italy |
|
Plemazol |
Egypt |
|
Pramadin |
Venezuela |
|
Pramotil |
Mexico |
|
Primperan |
Belgium, Colombia, Denmark, Ecuador, Egypt, Finland, France, Greece, Luxembourg, Malaysia, Mexico, Morocco, Netherlands, Peru, Portugal, Saudi Arabia, Spain, Sweden, Switzerland, Tunisia, Turkey, United Arab Emirates, Venezuela |
|
Primsel |
Turkey |
|
Reglan |
Slovenia, United States of America |
|
|
 |
 |
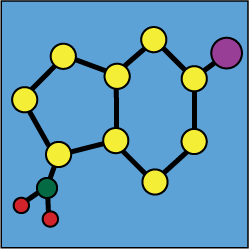
Stability of mixtures : Metoclopramide hydrochloride |
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
0,5 mg/ml |
|
22°C |
| 42 |
 |
|
 |
1495

|
 |
 |
0,5 mg/ml |
|
4°C |
| 189 |
 |
|
 |
1495

|
 |
 |
0,5 mg/ml |
|
22°C |
| 14 |
 |
|
 |
1495

|
 |
 |
0,5 mg/ml |
|
4°C |
| 35 |
 |
|
 |
1495

|
 |
 |
25 mg/ml |
|
25°C |
| 4 |
 |
|
 |
2179

|
 |
 |
0,5 mg/ml |
|
22°C |
| 42 |
 |
|
 |
1495

|
 |
 |
0.15 mg/ml |
|
32°C |
| 48 |
 |
|
 |
930

|
 |
 |
0,5 mg/ml |
|
4°C |
| 189 |
 |
|
 |
1495

|
 |
 |
0.15 mg/ml |
|
32°C |
| 48 |
 |
|
 |
930

|
 |
 |
0,1 mg/ml |
|
4°C |
| 32 |
 |
|
 |
3082

|
 |
 |
0,7 mg/ml |
|
32°C |
| 10 |
 |
|
 |
1405

|
 |
 |
0,5 mg/ml |
|
22°C |
| 42 |
 |
|
 |
1495

|
 |
 |
2,5 mg/ml |
|
23°C |
| 24 |
 |
|
 |
815

|
 |
 |
0,5 mg/ml |
|
25°C |
| 96 |
 |
|
 |
2166
|
 |
 |
0,5 mg/ml |
|
25°C |
| 96 |
 |
|
 |
2166
|
 |
 |
0,5 mg/ml |
|
4°C |
| 189 |
 |
|
 |
1495

|
 |
 |
2,5 mg/ml |
|
4°C |
| 24 |
 |
|
 |
815

|
 |
 |
0,5 mg/ml |
|
4°C |
| 96 |
 |
|
 |
2166
|
 |
 |
0,86 mg/ml |
|
20°C-23°C |
| 4 |
 |
|
 |
1026

|
 |
 |
1,6 mg/ml |
|
25°C |
| 48 |
 |
|
 |
3779

|
 |
 |
0,86 mg/ml |
|
20°C-23°C |
| 4 |
 |
|
 |
1026

|
|
|