| Type : |
Poster |

4548Ko
|
|
| Research teams : |
Toronto - Sunnybrook Health Sciences Center |
| Authors : |
Tyono I, Law S, Charbonneau L.F, Walker S.E. |
| Title : |
Stability of Azacitidine Solutions In Sterile Water for Injection. |
| Reference : |
Prof Practice Conference - Canadian Society of PH 2019 |
|
| Level of Evidence : |
|
| Physical stability : |
|
| Chemical stability : |
|
| Other methods : |
|
| Comments : |
|
List of drugs
 Azacitidine Azacitidine
|
 |
 |
 |
 |
10 & 25 mg/ml |
25°C |
 |
| 2 |
 |
|
|
 |
 |
 |
10 & 25 mg/ml |
-20°C |
 |
| 21 |
 |
|
|
 |
 |
 |
10 & 25 mg/ml |
25°C |
 |
| 4 |
 |
|
|
 |
 |
 |
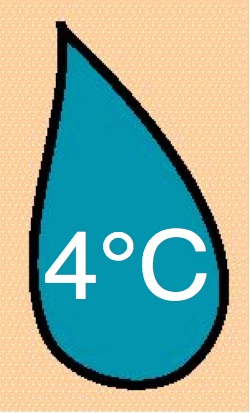
10 & 25 mg/ml |
4°C |
 |
| 24 |
 |
|
 |
 |
 |
 |
10 & 25 mg/ml |
-20°C |
 |
| 21 |
 |
|
|
 |
 |
 |
10 & 25 mg/ml |
4°C |
 |
| 24 |
 |
|
 |
|
|